SOLUTION_SPECIES
This keyword data block is
used to define chemical reaction, log K , and
activity-coefficient parameters for each aqueous species. In addition,
parameters may be defined for each species that are used to calculate specific
conductance, multicomponent diffusion, density, and enrichment in the diffuse
layer of surfaces. Normally, this data block is included in the database file
and only additions and modifications are included in the input file.
Example data block
Line 0: SOLUTION_SPECIES
Line 1: CO3-2 + H+ = HCO3-
Line 2: log_k 10.329
Line 3: delta_h -3.561 kcal
Line 4: -analytic 107.8871 0.03252849 -5151.79 -38.92561 563713.9
Line 5: -gamma 5.4000 0.0000
Line 6: -dw 1.18e-9
Line 7: -Vm 8.615 0 -12.21 0 1.667 0 0 264 0 1
Line 7:-Vm 10.26 -2.92 -12.58 -0.241 2.23 0 -5.49 320 2.83e-2 1.144
Line 8: -Millero 21.07 0.185 -0.002248 2.29 \ -0.006644 -3.667e-06
Line 1a: H2O = OH- + H+
Line 4a: -a_e -283.971 -0.05069842 13323.0 102.24447 -1119669.0
Line 4a:-a_e 293.29227 0.1360833 -10576.913 -123.73158 0 -6.996455e-5
Line 5a: -gamma 3.5000 0.0000Line 1b: D2O = D2O
Line 2a: log_k 0
Line 9: -activity_water
Line 1c: OH- + HDO = OD- + H2O
Line 2b: log_k -0.301029995663
Line 10: -add_logk Log_alpha_D_OH-/H2O(l) 1.0
Line 5b: -gamma 3.5000 0.0000
Line 1d: Cl- = Cl-
Line 2c: log_k 0
Line 11: -llnl_gamma 3.0000
Line 1e: 2H2O = O2 + 4H+ + 4e-
Line 2d: log_k -85.9951
Line 12: -co2_llnl_gamma
Line 1f: Cs+ = Cs+
Line 2d: log_k 0
Line 13: -erm_ddl 2.1
Line 1g: HS- = S2-2 + H+
Line 2e: log_k -14.528
Line 14: -no_check
Line 15: -mole_balance S(-2)2
Line 1h: H+ = H+Line 5b: -gamma 9.0 0Line 16: -viscosity 9.35e-2 -8.31e-2 2.487e-2 4.49e-4 2.01e-2 1.570 Line 6a: -dw 9.31e-9 838 16.315 0 2.376 24.01 0
Explanation
Keyword for the data block. No other data are input on the keyword
line.
Association reaction for aqueous species. The defined species must
be the first species to the right of the equal sign. The association reaction
must precede any identifiers related to the aqueous species. The association
reaction is an identity reaction for each primary master species.
log_k --Identifier for log K at 25 °C. Optionally, -log_k ,
logk , -l [
og_k ], or -l [ ogk ].
log K --Log K at 25 °C
for the reaction. Log K must be 0.0 for primary master species.
Default is 0.0.
Line 3: delta_h enthalpy,
[ units ]
delta_h --Identifier for enthalpy of reaction at 25 °C. Optionally, -delta_h
, deltah , -d
[ elta_h ], or -d [
eltah ].
enthalpy --Enthalpy
of reaction at 25 °C for the reaction. Default is 0.0 kJ/mol.
units --Default units are
kilojoules per mole. Units may be calories, kilocalories, joules, or kilojoules
per mole. Only the energy unit is needed (per mole is assumed) and
abbreviations of these units are acceptable. Default units are kJ/mol. Explicit
definition of units for all enthalpy values is recommended. The enthalpy of
reaction is used in the Van’t Hoff equation to determine the temperature
dependence of the equilibrium constant. Internally, all enthalpy calculations
are performed with the units kJ/mol.
Line 4: -analytic A 1
, A 2 , A 3 , A
4 , A 5 , A 6
-analytic
--Identifier for coefficients for an analytical expression for the temperature
dependence of log K . Optionally, analytical_expression , a_e ,
ae , -a [ nalytical_expression
], -a [ _e ], -a [ e
].
A 1
, A 2 , A 3 , A
4 , A 5 , A 6 --Six values
defining log K as a function of temperature in the expression 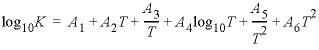 , where T
is in kelvin.
, where T
is in kelvin.
Line 5: -gamma Debye-Hückel
a, Debye-Hückel b
-gamma --Indicates
activity-coefficient parameters are to be entered. If -gamma
is entered, then the equation from WATEQ (Truesdell and Jones, 1974) is used, 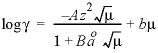 . In this equations,
. In this equations, ![]() is the activity coefficient,
is the activity coefficient, ![]() is ionic strength, and A and B are constants at
a given temperature. If -gamma is not input for a species,
then for a charged species the Davies equation is used to calculate the
activity coefficient:
is ionic strength, and A and B are constants at
a given temperature. If -gamma is not input for a species,
then for a charged species the Davies equation is used to calculate the
activity coefficient:
![]() ; for an
uncharged species the following equation is used:
; for an
uncharged species the following equation is used: ![]() . Optionally, -g
[ amma ].
. Optionally, -g
[ amma ].
Debye-Hückel a --Ion-size parameter ![]() in the
WATEQ activity-coefficient equation.
in the
WATEQ activity-coefficient equation.
Debye-Hückel b --Parameter b in the WATEQ activity-coefficient
equation.
Line 6: -dw Dw(25C) dw_T a a2 visc a3 a_v_dif
-dw --Identifier for tracer diffusion coefficient of the species. Tracer
diffusion coefficients are used in the multicomponent diffusion calculation in TRANSPORT
and in calculating the specific conductance of a solution (Basic function SC).
Default is 0 m 2 /s (square meter per second) if -dw is not included. Optionally, dw or -dw .
Dw(25C)—Tracer diffusion
coefficient for the species at 25 °C, m 2 /s.
dw_T—Temperature
dependence for diffusion coefficient.
a— Debye-Huckel ion size for correcting
the ionic strength dependent contribution of the species to the specific
conductance (SC).
a2— Exponent for the volume correction of a in the SC calculation.
Visc— Exponent for the viscosity
correction in the SC calculation.
a3— Ionic strength exponent, or a flag (see Notes).
A_v_dif— Exponent for
(viscosity_0_tc/viscosity), for correcting the tracer diffusion coefficient in
TRANSPORT calculations.
Line 6: -dw
diffusion coefficient damp a1 a2
-dw
--Identifier for tracer diffusion coefficient. Tracer diffusion
coefficients are used in the multicomponent diffusion calculation in TRANSPORT
and in calculating the specific conductance of a solution (Basic function SC).
Default is 0 m 2 /s (square meter per second) if -dw is not included. Optionally, dw
or -dw .
diffusion coefficient—Tracer
diffusion coefficient for the species at 25 °C, m 2 /s.
damp
--Damping parameter for
the temperature effect on viscosity on the diffusion coefficient. Dw(TK) =
D * exp(damp / TK - damp / 298.15) * TK * 0.89 / (298.15 * viscos), where Dw is the diffusion coefficient at temperature TK kelvin; D
is the diffusion coefficient defined by the first parameter; damp is the
damping factor; and viscos is the viscosity of the solution.
a1
--Parameter for the
ionic strength effects on the diffusion coefficient of ions in
electro-migration. Dw(I) = Dw(TK)
* exp(a1 * DH_A * |z| * I^0.5 / (1 + DH_B * I^0.5 * a2 / (1 + I^0.75))), where Dw(I) is the diffusion coefficient corrected for
temperature and ionic strength; Dw(TK) is the
diffusion coefficient corrected for temperature; a1 is the third parameter
defined in -dw; DH_A is the Debye-Huckel
A parameter; z is the absolute value of the charge of the aqueous species; I is
the ionic strength; DH_B is the Debye-Huckel B
parameter; and a2 is the fourth parameter defined in -dw.
a2--Parameter for the ionic strength effects on the diffusion
coefficient of ions in electro-migration in the equation above.
Line 7: -Vm a1, a2,
a3, a4, W, ![]() i1, i2, i3, i4
i1, i2, i3, i4
-Vm --Identifier for parameters used to calculate the specific volume
(cm 3 /mol) of aqueous species with a Redlich-type equation (see
Redlich and Meyer, 1964). As explained in the following Notes section, the volume of species i is calculated, by convention relative to the
reference volume of H + of 0, as ![]() , where the first term of the right-hand side,
, where the first term of the right-hand side, ![]() , is the specific volume at
infinite dilution, and the second and third terms are functions of the ionic
strength
, is the specific volume at
infinite dilution, and the second and third terms are functions of the ionic
strength ![]() and the ion-size parameter in the extended Debye-Hückel equation,
and the ion-size parameter in the extended Debye-Hückel equation, ![]() and i1, i2, i3 and i4.
and i1, i2, i3 and i4.
The specific volume at infinite dilution is parameterized with
SUPCRT92 formulas (Johnson and others, 1992):
where 41.84 transforms cal mol -1
bar -1 (calorie per mole per bar) into cm 3 /mol, P
b is pressure in bar, T K is temperature in kelvin, W x
Q Born is the Born volume, calculated from W and the pressure
dependence of the dielectric constant of water.
The second term contains A v , the Debye-Hückel limiting slope, which is calculated as a function of
temperature and pressure, and the extended Debye-Hückel
equation (see the Notes).
The coefficient ![]() is calculated as
is calculated as ![]() .
.
a1, a2, a3, a4, W, ![]() i1, i2, i3, i4
--Numerical values for parameters a1 to a4 (cal mol
-1 bar -1 , cal/mol (calorie per
mole), cal K mol -1 bar -1
[calorie kelvin per mole per bar), cal K mol -1
[calorie kelvin per mol], respectively), the Born coefficient W (cal/mol), the Debye-Hückel ion-size
parameter
i1, i2, i3, i4
--Numerical values for parameters a1 to a4 (cal mol
-1 bar -1 , cal/mol (calorie per
mole), cal K mol -1 bar -1
[calorie kelvin per mole per bar), cal K mol -1
[calorie kelvin per mol], respectively), the Born coefficient W (cal/mol), the Debye-Hückel ion-size
parameter ![]() (10-10 m), and i1 (cm 3 /mol), i2 (cm 3 K
mol -1 ), i2 (cm 3 K -1 mol -1 )
and i4 (-), used in the equation for calculating the conventional specific
volume of a solute species.
(10-10 m), and i1 (cm 3 /mol), i2 (cm 3 K
mol -1 ), i2 (cm 3 K -1 mol -1 )
and i4 (-), used in the equation for calculating the conventional specific
volume of a solute species.
Line 8: -Millero
a, b, c, d, e, f
-Millero --Alternative formulation for calculating the specific volume for
the aqueous species (Millero, 2000) by convention relative
to the volume of H + of 0 at ionic strength of 0. The specific
volume for species i is calculated according
to the formula ![]() , where
, where ![]() is the specific volume at infinite dilution; A v is the
Debye-Hückel limiting slope, and I is the
ionic strength. The volume at infinite dilution is parameterized as
is the specific volume at infinite dilution; A v is the
Debye-Hückel limiting slope, and I is the
ionic strength. The volume at infinite dilution is parameterized as ![]() and the
coefficient
and the
coefficient ![]() is parameterized as
is parameterized as ![]() , where T is °C. If both - Vm and - Millero
are defined for a species, the numbers from - Vm are used. Warning: the applicability of the Millero formulas is limited to T < 50 °C, and the
calculated densities may be incorrect at ionic strengths > 1.0 except for
NaCl solutions. Optionally, Millero
or -Mi [ llero ].
, where T is °C. If both - Vm and - Millero
are defined for a species, the numbers from - Vm are used. Warning: the applicability of the Millero formulas is limited to T < 50 °C, and the
calculated densities may be incorrect at ionic strengths > 1.0 except for
NaCl solutions. Optionally, Millero
or -Mi [ llero ].
a, b, c, d, e, f
--Numerical values for parameters a to f in the specific volume equation.
-activity_water --Identifier indicates that the species is an isotopic form of
water. The activity coefficient for the species is such that its activity is
equal to mole fraction in solution. Optionally, activity_water
or -ac [ tivity_water ].
Line 10: -add_logk named
log K, coefficient
-add_logk --Identifier defining an additional term for the equilibrium
constant of the species. The identifier is used primarily in defining the
equilibrium constant for isotopic species that require the addition of an
isotopic fractionation factor. Optionally, add_logk , add_log_k , -ad [
d_logk ] or -ad [ d_log_k ].
named log K --Name of an expression
defined in a MIX_EQUILIBRIUM_PHASES
data block.
coefficient --Coefficient
for the expression named log K ; the value of the expression is multiplied by coefficient
and added to the log K for the species.
-llnl_gamma --Identifier for the hard-core diameter in the expression for the
activity coefficient in the Lawrence Livermore aqueous model; this identifier
can be used only with the Lawrence Livermore National Laboratory aqueous model ( llnl.dat ). Optionally, llnl_gamma
or -ll [ nl_gamma ].
diameter --Hard-core diameter for the
species.
-co2_llnl_gamma --The
activity coefficient for carbon dioxide is used as the activity coefficient for
this uncharged species; this identifier can be used only with the Lawrence
Livermore National Laboratory aqueous model ( llnl.dat
). Optionally, co2_llnl_gamma or -co [
2_llnl_gamma ].
-erm_ddl --Identifier for the enrichment factor for a species in the
diffuse double layer of surfaces calculated with the -Donnan
identifier in the SURFACE data
block. Optionally, erm_ddl or -e
[ rm_ddl ].
factor --Enrichment factor. Default
is 1.0 (unitless).
-no_check --Indicates the reaction equation should not be checked for
charge and elemental balance. Generally, equations should be checked for charge
and elemental balance. The only exceptions might be polysulfide species that
assume equilibrium with a solid phase; this assumption has the effect of
removing solid sulfur from the mass-action equation. By default, all equations
are checked. However, the identifier -mole_balance
is needed to ensure that the proper number of atoms of each element are
included in mole-balance equations (see -mole_balance ).
Optionally, no_check or -n
[ o_check ].
Line 15: -mole_balance formula
-mole_balance --Indicates the stoichiometry of the species will be defined
explicitly. Optionally, mole_balance , mass_balance ,
mb , -m [ ole_balance
], -mass_balance , or -m
[ b ].
formula --Chemical formula defining
the stoichiometry of the species. Normally, both the stoichiometry and
mass-action expression for the species are determined from the chemical
equation that defines the species. Rarely, it may be necessary to define the
stoichiometry of the species separately from the mass-action equation. The
polysulfide species provide an example. These species are usually assumed to be
in equilibrium with native sulfur. The activity of a pure solid is 1.0, and
thus the term for native sulfur does not appear in the mass-action expression
(Line 1g). The S 2 - species contains two atoms of
sulfur, but the chemical equation indicates it is formed from species
containing a total of one sulfur atom. The -mole_balance
identifier is needed to give the correct stoichiometry. Note that unlike all
other chemical formulas used in PHREEQC, the valence state of the element can
and should be included in the formula of Line 15. The example indicates that
the polysulfide species will be summed into the S(-2)
mole-balance equation.
Line 16: -viscosity
b0,
b1, b2, d1, d2, d3, tan
- viscosity --Defines viscosity parameters for the species. Optionally, viscosity
or -vi[scosity].
b0,
b1, b2, d1, d2, d3, tan—See
explanation in the following Notes section for the equations and parameters
used for viscosity.
Notes
Line 1 must be entered first in the definition of a species.
Additional sets of lines (Lines 1-7 as needed) may be added to define all of the aqueous species. A log K should be
defined for each species with either log_k
(Line 2) or -analytical_expression
(Line 4); the default of 0.0 is not meaningful for most association reactions.
In this Example data block, the following types of aqueous species are defined:
(a) a primary master species, SO 4 -2 , for which the reaction
is an identity reaction and log K is 0.0; (b) a secondary master
species, HS - , for which the reaction contains electrons; (c) an
aqueous species that is not a master species, OH - ; and (d) an
aqueous species for which the chemical equation does not balance, S 2
-2 . If an activity coefficient of 1 is needed for a species, use -gamma
1e5 0 in Line 5.
The tracer diffusion coefficient is for a trace concentration of
the solute species in pure water. Usually, it is determined by measuring the specific
conductance of solutions at various concentrations and extrapolating to zero
concentration (Robinson and Stokes, 2002). The molar conductivity of a solute
species and its diffusion coefficient are related by ![]() , where
, where ![]() is the molar conductivity (S/m
/ (mol/m3) equals S m 2 mol -1 ) (siemens square meter
per mole), zi is the charge number (unitless) of species i,
F is Faraday's constant (C/mol, coulomb per mole), R is the gas constant (J
K-1mol-1, joule per kelvin per mole), T is the absolute temperature (K), and Dw, i is the diffusion
coefficient (m2/s). PHREEQC calculates the specific conductance of a solution
by summing the product of the specific conductivity and the molal concentration
of all the species in solution, while correcting the molal concentration with
an electrochemical activity coefficient that is derived from a combination of
Kohlrausch’s law and the Debye-Hückel equation as
explained in http://www.hydrochemistry.eu/exmpls/sc.html (accessed June 25,
2012).
is the molar conductivity (S/m
/ (mol/m3) equals S m 2 mol -1 ) (siemens square meter
per mole), zi is the charge number (unitless) of species i,
F is Faraday's constant (C/mol, coulomb per mole), R is the gas constant (J
K-1mol-1, joule per kelvin per mole), T is the absolute temperature (K), and Dw, i is the diffusion
coefficient (m2/s). PHREEQC calculates the specific conductance of a solution
by summing the product of the specific conductivity and the molal concentration
of all the species in solution, while correcting the molal concentration with
an electrochemical activity coefficient that is derived from a combination of
Kohlrausch’s law and the Debye-Hückel equation as
explained in http://www.hydrochemistry.eu/exmpls/sc.html (accessed June 25,
2012). The tracer diffusion coefficient is corrected for the temperature T
(K) of the solution by D ’ w,i = (D w,i
)298 × ![]()
× ![]()
, where η is the
viscosity of water (Atkins and de Paula, 2002).
The tracer diffusion
coefficient is corrected for the temperature T(K) of the solution by Dw(TK) = Dw(298) * exp(dw_T / TK - dw_T / 298.15) *
viscos_0_25 / viscos_0_tc, where viscos_0_25 and viscos_0_tc are the
viscosities of water at 25 and tc (the temperature of
the solution, oC), respectively, calculated with the
polynomials of Huber et al., 2009, J. Phys. Chem. Ref. Data, Vol. 38, 101-125.
Optionally, for TRANSPORT calculations, the diffusion coefficient can be
corrected for the viscosity of the solution with the a_v_dif
parameter: Dw(TK) = Dw(TK) * (viscos_0_tc /
viscos)^a_v_dif, where viscos is the T,P,I-dependent
viscosity of the solution.
The specific conductance is
calculated with a corrected Dw(TK), using the parameters defined in line 6a:
Dw(TK) = Dw(TK) * viscos_0_tc /
viscos)^visc (visc = 2.376 for H+ in line 6a)
If a3 > 5 or a3 = 0 or not defined, then
the Debye-Hückel ka = DH_B * a * (1 + (vm - v0))^a2 * mu^0.5 (DH_B is the Debye-Hückel B parameter, a = 16.315 for H+, vm
= is the T,P,I dependent volume of the ion, v0 is the T,P dependent volume at I
= 0, a2 = 0 for H+, the reference for the molar volume of the ions, with vm = vo = 0)
If a3 = -10, then ka = DH_B * a * mu^a2 (Define a3 = -10,
not used in this database.) (a3 = 24.01 for H+, a flag to indicate the use of
the Debye-Huckel-Onsager-Falkenhagen formulas.)
If -3 < a3 < 4, then ka = DH_B * a2 *
mu^0.5 / (1 + mu^a3), Appelo, 2017, Cement and Concrete Research, 101, 102-113.
And Dw(I) = Dw(TK) * exp(-a * DH_A * z * sqrt_mu
/ (1 + ka)) (used for Sr+2 in PHREEQC.DAT).
The Dw and a_v_dif
can be set in a USER_ program with setdiff_c("name", Dw, a_v_dif), for example: 10
print setdiff_c("H+", 9.31e-9, 1).
If -Vm is defined, the
specific volume of species i is calculated,
by convention relative to the reference volume of H + of 0, as
where the first term of the right-hand side, ![]() , is the volume at infinite
dilution; and the second and third terms are functions of the ionic strength
, is the volume at infinite
dilution; and the second and third terms are functions of the ionic strength ![]() .
.
In the second term, z is the charge number of the species, A
v is the Debye-Hückel limiting slope,
with the Debye length factor, 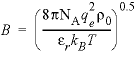 (1/cm)(kg/mol) 0.5 , Avogadro’s number N A =
6.022 × 10 23 molecules per mole, the electron charge q
e = 4.803 × 10 -10 esu
(electrostatic unit of charge), the density of pure water ñ 0
(g/cm 3 ), the relative dielectric constant å r
, the Boltzmann constant k B = 1.38 × 10 -16 erg/K
(erg per kelvin), the temperature T (K), the pressure P (atm), and the
compressibility of pure water ê 0 (atm -1
). PHREEQC calculates the relative dielectric constant as a function of
temperature and pressure, as well as its pressure dependence, according to
Bradley and Pitzer (1979), and the density of pure water along the saturation
line with equation 2.6 of Wagner and Pruss (2002) and at higher pressures and
temperatures with interpolation functions based on IAPWS (International
Association for the Properties of Water and Steam)
(http://www.nist.gov/srd/upload/NISTIR5078-Tab3.pdf) or with the IF97 (http://www.iapws.org/release.htm)
polynomial for region 1 (273 < T < 623 °C, P sat < P <
100 MPa, megapascal). The Bradley and Pitzer equations also are used to
calculate
(1/cm)(kg/mol) 0.5 , Avogadro’s number N A =
6.022 × 10 23 molecules per mole, the electron charge q
e = 4.803 × 10 -10 esu
(electrostatic unit of charge), the density of pure water ñ 0
(g/cm 3 ), the relative dielectric constant å r
, the Boltzmann constant k B = 1.38 × 10 -16 erg/K
(erg per kelvin), the temperature T (K), the pressure P (atm), and the
compressibility of pure water ê 0 (atm -1
). PHREEQC calculates the relative dielectric constant as a function of
temperature and pressure, as well as its pressure dependence, according to
Bradley and Pitzer (1979), and the density of pure water along the saturation
line with equation 2.6 of Wagner and Pruss (2002) and at higher pressures and
temperatures with interpolation functions based on IAPWS (International
Association for the Properties of Water and Steam)
(http://www.nist.gov/srd/upload/NISTIR5078-Tab3.pdf) or with the IF97 (http://www.iapws.org/release.htm)
polynomial for region 1 (273 < T < 623 °C, P sat < P <
100 MPa, megapascal). The Bradley and Pitzer equations also are used to
calculate 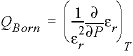 , which is a part of
, which is a part of ![]() . The specific volumes are used to derive the volume changes of
reactions, and hence, the pressure dependency of reaction constants for
species, and the pressure dependent solubilities of minerals and gases. The
volumes also are used for calculating the density of solutions in PHREEQC as
implemented by Vincent Post (Free University, Amsterdam, Netherlands, written commun., 2009) based on the work of Millero
(2000). The parameters, entered with the identifier -Vm in the phreeqc.dat and pitzer.dat
databases and commented with “# supcrt modified”, were obtained by least squares fitting of the specific
volumes of salts in aqueous solution, compiled by Laliberté
(2009), supplemented with data at lower concentrations (omitted by Laliberté (2009)) and at higher temperatures. In the
databases, the ion-size parameter for anions in the extended Debye-Hückel equation,
. The specific volumes are used to derive the volume changes of
reactions, and hence, the pressure dependency of reaction constants for
species, and the pressure dependent solubilities of minerals and gases. The
volumes also are used for calculating the density of solutions in PHREEQC as
implemented by Vincent Post (Free University, Amsterdam, Netherlands, written commun., 2009) based on the work of Millero
(2000). The parameters, entered with the identifier -Vm in the phreeqc.dat and pitzer.dat
databases and commented with “# supcrt modified”, were obtained by least squares fitting of the specific
volumes of salts in aqueous solution, compiled by Laliberté
(2009), supplemented with data at lower concentrations (omitted by Laliberté (2009)) and at higher temperatures. In the
databases, the ion-size parameter for anions in the extended Debye-Hückel equation, ![]() is equal to 0, and for
cations equal to the Debye -Hückel a parameter that is entered with -gamma a. The
values defined with -Millero in some
(now obsolete) databases are, in principle, for the temperature range from 0 to
50 o C (Millero, 2000) and may be
incorrect for high ionic strengths except for solutions containing
predominantly alkali cations and chloride anions.
is equal to 0, and for
cations equal to the Debye -Hückel a parameter that is entered with -gamma a. The
values defined with -Millero in some
(now obsolete) databases are, in principle, for the temperature range from 0 to
50 o C (Millero, 2000) and may be
incorrect for high ionic strengths except for solutions containing
predominantly alkali cations and chloride anions.
The Lawrence Livermore National Laboratory aqueous model (Daveler
and Wolery, 1992) uses the following expression for the log (base 10) of an
activity coefficient:  , where
, where ![]() ,
, ![]() , and
, and ![]() are Debye-Hückel parameters that are
functions of temperature as defined in the LLNL_AQUEOUS_MODEL_PARAMETERS
data block; z i is the charge
number for species i,
are Debye-Hückel parameters that are
functions of temperature as defined in the LLNL_AQUEOUS_MODEL_PARAMETERS
data block; z i is the charge
number for species i, ![]() is the hard-core diameter, which is defined for an aqueous species
in the SOLUTION_SPECIES data block with the -llnl_gamma identifier; and I is the ionic
strength. The activity for an uncharged species in the Lawrence Livermore
National Laboratory aqueous model can be set to a function of temperature by
using the -co2_llnl_gamma identifier. The function of
temperature is defined by the -co2_coefs identifier in the LLNL_AQUEOUS_MODEL_PARAMETERS
data block.
is the hard-core diameter, which is defined for an aqueous species
in the SOLUTION_SPECIES data block with the -llnl_gamma identifier; and I is the ionic
strength. The activity for an uncharged species in the Lawrence Livermore
National Laboratory aqueous model can be set to a function of temperature by
using the -co2_llnl_gamma identifier. The function of
temperature is defined by the -co2_coefs identifier in the LLNL_AQUEOUS_MODEL_PARAMETERS
data block.
The enrichment factor entered with -erm_ddl
multiplies the concentration that is calculated with the Boltzmann equation for
the Donnan space on a charged surface. With this factor, the concentrations in
the Donnan space are calculated as:
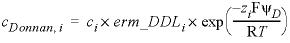 , (4)
, (4)
where cDonnan, i
is the concentration of species i in the Donnan pore
space (mol/L), ci is the concentration in the free (uncharged) solution, erm_DDLi is an enrichment factor (unitless) that can be
defined in keyword SOLUTION_SPECIES,
zi is charge number (unitless), F is the Faraday constant (96485 JV-1eq-1,
joule per volt per equivalent), ψD is the
potential of the Donnan volume (V, volt), R is the gas constant (8.314
JK-1mol-1), and T is the absolute temperature (K). The potential ψD is adapted to let the charge of the Donnan volume
counterbalance the surface charge:
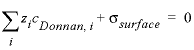 , (5)
, (5)
where σsurface is the surface
charge (eq/L, equivalent per liter). The enrichment factor is useful for
modeling the relative enrichment or depletion of equally charged species in the
electrostatic layer on a charged surface, which is related to enhanced
complexation in a low dielectric permittivity medium (Appelo and others, 2010).
By default, equation checking for charge and elemental balance is
in force for each equation that is processed. Checking can only be disabled by
using -no_check for each equation
that is to be excluded from the checking process.
The viscosity of the
solution at P, T is now calculated and printed in the output file, and
can be retrieved in Basic programs with the function viscos (in
previous versions, viscos returned the viscosity of pure water at P, T).
The calculation uses a modified Jones-Dole equation which sums the
contributions of individual solutes:
η / η0 = 1 + A √(0.5 ∑ zimi)
+ ∑ fan (Bimi
+ Dimini),
where η is the viscosity of the solution (mPa s), η0
idem of pure water at the temperature and pressure of the solution, mi
is the molality of species i, made
dimensionless by dividing by 1 molal, and zi is the absolute
charge number. A is derived from Debye-Hückel
theory, and fan, B, D and n
are coefficients that incorporate volume, ionic strength and temperature
effects.
The coefficients are:
B = b0 + b1 exp(-b2 tC)
where b0..2 are coefficients, and tC
is the temperature in ºC. The temperature is limited to 200°C.
fan = (2 - tan Van
/ VCl-)
for anions, with tan a coefficient and Van
the P, T and I dependent, apparent
volume of the anion relative to the one of Cl-, which is used as
reference species. For cations, fan = 1 and tan
needs not be defined.
D = d1 exp(-d2
tC )
where d1, 2 are coefficients.
n = ((1 + fI)d3 + ((zi2
+ zi) / 2 · mi)d3
/ (2 + fI)
where fI averages ionic strength
effects and d3 is a coefficient.
The coefficients are fitted on measured viscosities of binary solutions, for
example for H+:
SOLUTION_SPECIES
H+ = H+
-viscosity 9.35e-2 -8.31e-2 2.487e-2 4.49e-4 2.01e-2 1.570
0
# b0
b1 b2
d1
d2 d3 tan
Example problems
The keyword SOLUTION_SPECIES is used in example
problems 1, 7, 9, 14, 15, and 21 and in all databases.

