RATES
This keyword data block
is used to define mathematical rate expressions for kinetic reactions. General
rate formulas are defined in the RATES data block and specific
kinetic parameters for batch reaction or transport are defined in the KINETICS data block.
Example
data block
Line 0: RATES
Line 1: Calcite
Line 2: -start
Basic: 1 rem M = current number of moles of calcite
Basic: 2 rem M0 = number of moles of calcite initially present
Basic: 3 rem PARM(1) = A/V, cm^2/L
Basic: 4 rem PARM(2) = exponent for M/M0
Basic: 10 si_cc = SI("Calcite")
Basic: 20 if (M <= 0 and si_cc < 0) then goto 200
Basic: 30 k1 = 10^(0.198 - 444.0 / TK )
Basic: 40 k2 = 10^(2.84 - 2177.0 / TK)
Basic: 50 if TC <= 25 then k3 = 10^(-5.86 - 317.0 / TK )
Basic: 60 if TC > 25 then k3 = 10^(-1.1 - 1737.0 / TK )
Basic: 70 t = 1
Basic: 80 if M0 > 0 then t = M/M0
Basic: 90 if t = 0 then t = 1
Basic: 100 area = PARM(1) * (t)^PARM(2)
Basic: 110 rf = k1*ACT("H+")+k2*ACT("CO2")+k3*ACT("H2O")
Basic: 120 rem 1e-3 converts mmol to mol
Basic: 130 rate = area * 1e-3 * rf * (1 - 10^(2/3*si_cc))
Basic: 140 moles = rate * TIME
Basic: 200 SAVE moles
Line 3: -end
Line 1a: Pyrite
Line 2a: -start
Basic: 1 rem PARM(1) = log10(A/V, 1/dm)
Basic: 2 rem PARM(2) = exp for (M/M0)
Basic: 3 rem PARM(3) = exp for O2
Basic: 4 rem PARM(4) = exp for H+
Basic: 10 if (M <= 0) then goto 200
Basic: 20 if (SI("Pyrite") >= 0) then goto 200
Basic: 30 lograte = -10.19 + PARM(1) + PARM(2)*LOG10(M/M0)
Basic: 40 lograte = lograte + PARM(3)*LM("O2") + PARM(4)*LM("H+")
Basic: 50 moles = (10^lograte) * TIME
Basic: 60 if (moles > M) then moles = M
Basic: 200 SAVE moles
Line 3a: -end
Explanation
RATES
is the keyword for the data block. No other data are input on the keyword line.
Line
1: name of rate expression
name
of rate expression --Alphanumeric character string that
identifies the rate expression; no spaces are allowed.
-start
--Identifier marks the beginning of a Basic program by which the moles of
reaction for a time subinterval are calculated.
Basic:
numbered Basic statement
numbered
Basic statement --A valid Basic language statement that
must be numbered. The statements are evaluated in numerical order. The sequence
of statements must extrapolate the rate of reaction over the time subinterval
given by the internally defined variable TIME. There must be a statement “
SAVE expression ”, where the value of expression is
the moles of reaction that are transferred during time subinterval TIME.
Statements and functions that are available through the Basic interpreter are
listed in the section on the Basic interpreter. Parameters defined in the KINETICS data block also are
available through the Basic array PARM.
-end
--Identifier marks the end of a Basic program by which the number of moles of a
reaction for a time subinterval is calculated. Note the hyphen is required to
avoid a conflict with the keyword END .
Notes
A Basic interpreter
(David Gillespie, Synaptics, Inc., San Jose, Calif.,
written commun., 1997) distributed with the Linux
operating system (Free Software Foundation, Inc.) is embedded in PHREEQC. The
Basic interpreter is used during the integration of the kinetic reactions to
evaluate the moles of reaction progress for a time subinterval. A Basic program
for each kinetic reaction must be included in the input or database file. Each
program must stand alone with its own set of variables and numbered statement
lines. There is no conflict in using the same variable names or line numbers in
separate rate programs.
It is possible to
transfer data among rates with the special Basic statements PUT and GET (see The Basic Interpreter). The programs
are used to calculate the instantaneous rate of reaction and extrapolate that
rate for a time subinterval given by the variable “TIME” (calcite, line 140;
pyrite line 50). TIME is an internally generated and variable time substep, and its value cannot be changed. The total moles
of reaction must be returned to the main program with a SAVE command (line 200
in each example). Note that moles of reaction are returned, not the rate of the
reaction. Moles are counted positive when the solution concentration of the
reactant increases.
The first example
estimates the rate of calcite dissolution or precipitation on the basis of a
rate expression from Plummer and others (1978) (see also equations 101 and 106,
Parkhurst and Appelo, 1999). The forward rate is given by
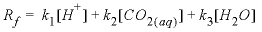 , (1)
, (1)
where
square brackets indicate activity and ![]() ,
,
![]() , and
, and ![]() are functions of temperature (Plummer and
others, 1978). In a pure calcite-water system with fixed
are functions of temperature (Plummer and
others, 1978). In a pure calcite-water system with fixed ![]() , the overall rate for calcite (forward
rate minus backward rate) is approximated by
, the overall rate for calcite (forward
rate minus backward rate) is approximated by
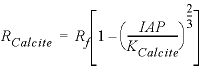 , (2)
, (2)
where
![]() is mmol cm -2
s -1 (millimole per square centimeter per second). Equation 2 is implemented in Basic for the
first example above. Explanations of the Basic lines for this rate expression
are given in table 5.
is mmol cm -2
s -1 (millimole per square centimeter per second). Equation 2 is implemented in Basic for the
first example above. Explanations of the Basic lines for this rate expression
are given in table 5.
The second example is
for the dissolution of pyrite in the presence of dissolved oxygen from
Williamson and Rimstidt (1994):
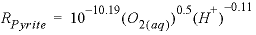 , (3)
, (3)
where
parentheses indicate molality. This rate is based on detailed measurements in
solutions of varying compositions and shows a square root dependence on the
molality of oxygen and a small dependence on pH. This
rate is applicable only for dissolution in the presence of oxygen and will be
incorrect near equilibrium when oxygen is depleted. Explanations of the Basic
lines for this rate expression are given in table 6.
Some special statements
and functions have been added to the Basic interpreter to allow access to
quantities that may be needed in rate expressions. These functions are listed
in The Basic Interpreter, table 8. Standard Basic statements
that are implemented in the interpreter are listed in The Basic Interpreter, table 7. Upper or lower case may be
used for statement, function, and variable names. String variable names must
end with the character “$”.
The PRINT command in
Basic programs is useful for debugging rate expressions. It can be used to
write quantities to the output file to check that rates are calculated
correctly. However, the PRINT command will write to the output file every time
a rate is evaluated, which may be many times per time step. The sequence of
information from PRINT statements in RATES
definitions may be difficult to interpret because of the automatic time-step
adjustment of the integration method.