Table 29. Input file for example 10.
TITLE Example 10.--Solid solution of strontianite and aragonite.
|
PHASES
|
Strontianite
|
SrCO3 = CO3-2 + Sr+2
|
log_k -9.271
|
Aragonite
|
CaCO3 = CO3-2 + Ca+2
|
log_k -8.336
|
END
|
SOLID_SOLUTIONS 1
|
Ca(x)Sr(1-x)CO3
|
-comp1 Aragonite 0
|
-comp2 Strontianite 0
|
-Gugg_nondim 3.43 -1.82
|
END
|
SOLUTION 1
|
-units mmol/kgw
|
pH 5.93 charge
|
Ca 3.932
|
C 7.864
|
EQUILIBRIUM_PHASES 1
|
CO2(g) -0.01265 10
|
Aragonite
|
SAVE solution 1
|
END
|
#
|
# Total of 0.00001 to 0.005 moles of SrCO3 added
|
#
|
USE solution 1
|
USE solid_solution 1
|
REACTION 1
|
SrCO3 1.0
|
.005 in 500 steps
|
PRINT
|
-reset false
|
-echo true
|
-user_print true
|
USER_PRINT
|
-start
|
10 sum = (S_S("Strontianite") + S_S("Aragonite"))
|
20 if sum = 0 THEN GOTO 110
|
30 xb = S_S("Strontianite")/sum
|
40 xc = S_S("Aragonite")/sum
|
50 PRINT "Simulation number: ", SIM_NO
|
60 PRINT "Reaction step number: ", STEP_NO
|
70 PRINT "SrCO3 added: ", RXN
|
80 PRINT "Log Sigma pi: ", LOG10 (ACT("CO3-2") * (ACT("Ca+2") + ACT("Sr+2")))
|
90 PRINT "XAragonite: ", xc
|
100 PRINT "XStrontianite: ", xb
|
110 PRINT "XCa: ", TOT("Ca")/(TOT("Ca") + TOT("Sr"))
|
120 PRINT "XSr: ", TOT("Sr")/(TOT("Ca") + TOT("Sr"))
|
130 PRINT "Misc 1: ", MISC1("Ca(x)Sr(1-x)CO3")
|
140 PRINT "Misc 2: ", MISC2("Ca(x)Sr(1-x)CO3")
|
-end
|
SELECTED_OUTPUT
|
-file ex10.sel
|
-reset false
|
-reaction true
|
USER_PUNCH
|
-head lg_SigmaPi X_Arag X_Stront X_Ca_aq X_Sr_aq mol_Misc1 mol_Misc2 \
|
mol_Arag mol_Stront
|
-start
|
10 sum = (S_S("Strontianite") + S_S("Aragonite"))
|
20 if sum = 0 THEN GOTO 60
|
30 xb = S_S("Strontianite")/(S_S("Strontianite") + S_S("Aragonite"))
|
40 xc = S_S("Aragonite")/(S_S("Strontianite") + S_S("Aragonite"))
|
50 REM Sigma Pi
|
60 PUNCH LOG10(ACT("CO3-2") * (ACT("Ca+2") + ACT("Sr+2")))
|
70 PUNCH xc # Mole fraction aragonite
|
80 PUNCH xb # Mole fraction strontianite
|
90 PUNCH TOT("Ca")/(TOT("Ca") + TOT("Sr")) # Mole aqueous calcium
|
100 PUNCH TOT("Sr")/(TOT("Ca") + TOT("Sr")) # Mole aqueous strontium
|
110 x1 = MISC1("Ca(x)Sr(1-x)CO3")
|
120 x2 = MISC2("Ca(x)Sr(1-x)CO3")
|
130 if (xb < x1 OR xb > x2) THEN GOTO 250
|
140 nc = S_S("Aragonite")
|
150 nb = S_S("Strontianite")
|
160 mol2 = ((x1 - 1)/x1)*nb + nc
|
170 mol2 = mol2 / ( ((x1 -1)/x1)*x2 + (1 - x2))
|
180 mol1 = (nb - mol2*x2)/x1
|
190 REM # Moles of misc. end members if in gap
|
200 PUNCH mol1
|
210 PUNCH mol2
|
220 GOTO 300
|
250 REM # Moles of misc. end members if not in gap
|
260 PUNCH 1e-10
|
270 PUNCH 1e-10
|
300 PUNCH S_S("Aragonite") # Moles aragonite
|
310 PUNCH S_S("Strontianite") # Moles Strontianite
|
-end
|
USER_GRAPH Example 10
|
-headings x_Aragonite x_Srontianite
|
-chart_title "Aragonite-Strontianite Solid Solution"
|
-axis_titles "Log(SrCO3 added, in moles)" "Log(Mole fraction of component)"
|
-axis_scale x_axis -5 1 1 1
|
-axis_scale y_axis -5 0.1 1 1
|
-connect_simulations true
|
-start
|
10 sum = (S_S("Strontianite") + S_S("Aragonite"))
|
20 IF sum = 0 THEN GOTO 70
|
30 xb = S_S("Strontianite")/ sum
|
40 xc = S_S("Aragonite")/ sum
|
50 PLOT_XY LOG10(RXN), LOG10(xc), line_w = 2, symbol_size = 0
|
60 PLOT_XY LOG10(RXN), LOG10(xb), line_w = 2, symbol_size = 0
|
70 rem
|
-end
|
END
|
#
|
# Total of 0.005 to 0.1 moles of SrCO3 added
|
#
|
USE solution 1
|
USE solid_solution 1
|
REACTION 1
|
SrCO3 1.0
|
.1 in 20 steps
|
END
|
#
|
# Total of 0.1 to 10 moles of SrCO3 added
|
#
|
USE solution 1
|
USE solid_solution 1
|
REACTION 1
|
SrCO3 1.0
|
10.0 in 100 steps
|
END
|
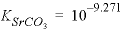 and
and 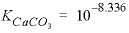 , and the Guggenheim parameters,
, and the Guggenheim parameters, 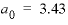 and
and 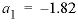 , are derived from Plummer and Busenberg (1987). The input file is shown in table 29. The PHASES data block defines the log
K
s for aragonite and strontianite and overrides any data for these minerals that might be present in the database file. The SOLID_SOLUTIONS data block defines the unitless Guggenheim excess free-energy parameters and the initial composition of the solid solution, which is zero moles of aragonite and strontianite. Initial solution 1 is defined to be a calcium bicarbonate solution. The solution is then equilibrated with aragonite at nearly 1 atm partial pressure of carbon dioxide and saved as the new composition of solution 1.
, are derived from Plummer and Busenberg (1987). The input file is shown in table 29. The PHASES data block defines the log
K
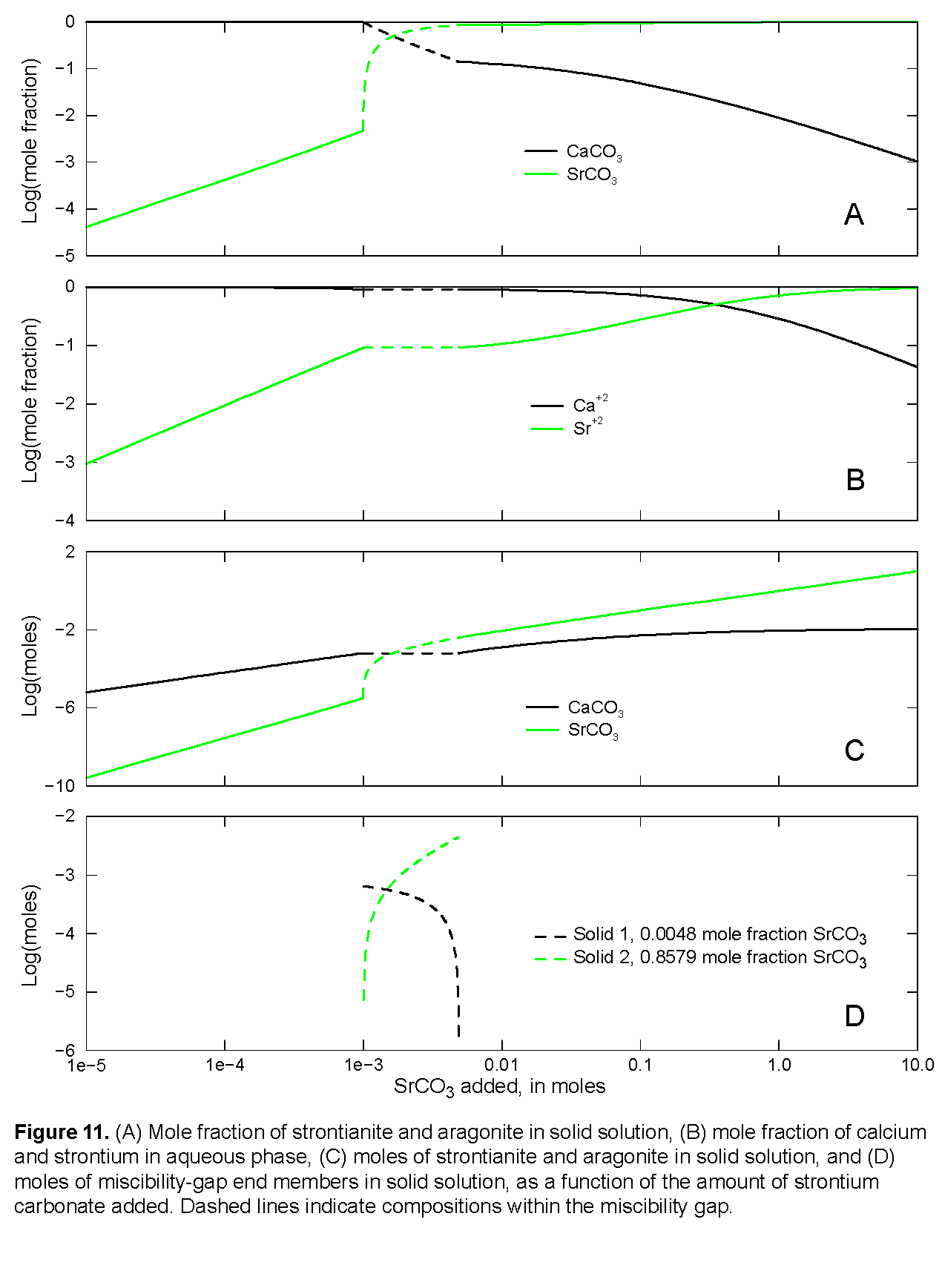
s for aragonite and strontianite and overrides any data for these minerals that might be present in the database file. The SOLID_SOLUTIONS data block defines the unitless Guggenheim excess free-energy parameters and the initial composition of the solid solution, which is zero moles of aragonite and strontianite. Initial solution 1 is defined to be a calcium bicarbonate solution. The solution is then equilibrated with aragonite at nearly 1 atm partial pressure of carbon dioxide and saved as the new composition of solution 1. 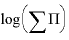 (log of the sum of the ion activity products), mole fractions of strontianite and aragonite, aqueous mole fractions of calcium and strontium, and the composition of the two solids that exist within the miscibility gap. The
(log of the sum of the ion activity products), mole fractions of strontianite and aragonite, aqueous mole fractions of calcium and strontium, and the composition of the two solids that exist within the miscibility gap. The