Analytical Procedures for SF6
SF6 is determined in the laboratory using a purge and trap gas chromatography procedure with an electron capture detector. This section provides an over view of the analysis procedure including extraction and measurement, calibration standards, accuracy and precision, and quality control.
Extraction and Measurement of SF6
The apparatus used for vacuum extraction of SF6 from groundwater (Figure A) is similar to the system described by Law and others (1994) and Busenberg and Plummer (2000). The apparatus consists of a 950 mL glass stripping vessel and various valves that control the flow of gases, water, and the vacuum. First, a vacuum is pulled into the 950 mL gas stripper cylinder. The stripper is isolated from the vacuum. The water sampling tube, a 3.2-mm outer diameter ss tube, is placed in the bottom of the bottle containing the water sample. A valve is opened and the vacuum pulls the water sample into the stripping cell. The water is sprayed through four small nozzles partially degassing the water. After the cell is filled to the 950 mL mark, the water inlet valve is closed, and ultra-pure N2 that was further purified by an activated charcoal trap is bubbled through the water, degassing the water. The stripped gases are dried and then collected on to the large trap of the Analytical System. After the stripping is completed (5 minutes) the stripper is isolated and pressurized with N2 which expels the water to waste after the water-to-waste valve is opened. After all the water is expelled, the valves V-A and V-B are closed. The stripper is re-evacuated and prepared to receive the next sample.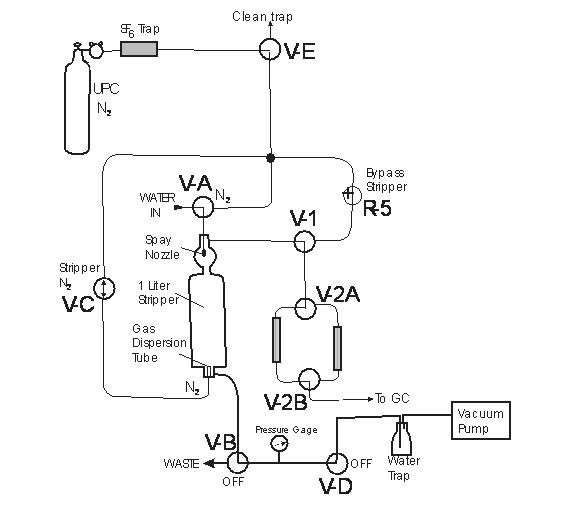
Figure A SF6 Extraction System
The analytical system for SF6 is described by Law and others, 1994; Busenberg and Plummer, 2000. The system was modified in 2009 when the HNU GC was replaced with a Shimadzu GC model GC-8A. Figure B shows the system that is currently used. The design of the gas distribution system is critical for obtaining good chromatograms from very small signals that are obtained from natural levels of SF6. UPC grade N2 is used throughout the system. The carrier gas is purified with a charcoal and a hydrocarbon-O2 trap. The pressure of the gas is controlled with four ultraclean stainless-steel diaphragm pressure regulators. Additional pressure and flow regulation was achieved by using dummy columns. These measures reduce background noise and increase the sensitivity of the system. For water samples, the stripped gas is trapped on a large trap immersed in an isopropyl alcohol-dry ice bath at about -70°C. The trapped SF6 is transferred into a small trap cooled in the isopropyl alcohol-dry ice bath by heating the large trap to 96°C. After the transfer is completed, the small trap is closed. The small trap is then heated to 96°C , and opened to inject the SF6 into the GC. The injection of the SF6 into the GC from the small trap greatly improves the detection limit of the system. The measurement is done by an electron capture detector (ECD) that is controlled by an integrator and a computer. SF6 Chromatography

Figure B SF6 Analytical System
Calibration of Analytical System
The SF6 system can measure a very wide range of concentrations. The injection loops used to standardize the system have volumes of 0.113, 0.3015, 0.512 and 15.2 cm3. Gas standards are used for daily calibration. The standards include a SIO Air Standard collected at Trinidad Head, California on March 12, 2008 with an SF6 partial pressure of 6.55 ± 0.0166 pptv. Also used daily is a Scott Specialty Gases standard with an SF6 partial pressure of 104 pptv.
Precision, Accuracy, Reporting Levels
All samples are analyzed in duplicate from two separate bottles. Standard deviations of 0.5 to 0.75 % are routinely obtained for repeated measurements of standards. The analytical precision of the water samples is about 20% at the minimum reporting level of 1.0 femtogram/liter and two to three percent at higher concentrations to a maximum reporting level of about 3.0 picograms per liter.
Quality Assurance and Control
Quality control water samples are prepared and analyzed daily. A sample is prepared in a one liter bottle by filling it with deionized water and bubbling laboratory air through the water with a pump and air stone. After bubbling for about 30 minutes, the sample is analyzed. Laboratory air is also analyzed. Using Henry's law and the temperature of the bubbled water, the partial pressure of SF6 in the water is compared to the concentration of SF6 in the laboratory air. They usually agree to within about 0.2 pptv. This procedure is an excellent verification of the stripping efficiency of the instrument. Gas blanks of the entire stripping and analytical system are also analyzed daily.
Analysis of SF6 in water references
Busenberg, E., and PlummerL.N., 2000, Dating young ground water with sulfur hexafluoride: Natural and anthropogenic sources of sulfur hexafluoride. Water Resources Research, 36, 3011-3030.
Law, C. S., Watson, A. J., and Liddicoat, M. I., 1994, Automated vacuum analysis of sulfur hexafluoride in seawater: derivation of the atmospheric trend (1979-1993) and potential as a transient tracer, Marine Chem., 48, 57-69.
Maiss, M., J. Ilmberger, A. Zenger, and K. O. Munnich, A SF6 tracer study of horizontal mixing in Lake Constance, Aquat. Sci., 56, 307-328, 1994.
Sliwka, I., and Lasa, J., 2000, Optimisation of the head-space method in measuring SF6 concentration in water. Chem. Anal. (Warsaw), 45, 59-72.
Wanninkhof, R., and Ledwell, J. R., 1991, Analysis of sulfur hexafluoride in seawater, Jour. Geophys. Res., 96C, 8733-8740.