Principles of Chromatography
The term chromatography is applied to separation techniques based on the partition of analytes between two phases in a dynamic system.
In gas chromatography, a gas is the mobile phase and a solid or liquid is the stationary phase. All analytes spend some time in the gaseous phase and in the stationary phase.
Retention is the time it takes for the analyte to elute from the column. The retention time is proportional to the amount of time the analyte spends in the stationary phase.
There are two types of columns:
- Packed columns are large diameter stainless steel or glass tubes (3.2-6.3 mm) filled with a solid stationary phase. Often a liquid coating is applied onto the solid phase. The lengths of the columns are about 1 to 5 m.
- Capillary glass-columns are thin (0.53-0.1 mm) and normally have a liquid stationary phase. Capillary columns with a solid porous layer are available (porous-layer open tabular, PLOT columns). Capillary columns are usually 15 to 100 m long.
Capillary columns are more difficult to use, are easily damaged by oxygen, and have lower precision than packed columns, however, they are more efficient and more compounds can be identified and quantified.
Sample SF6 Chromatogram
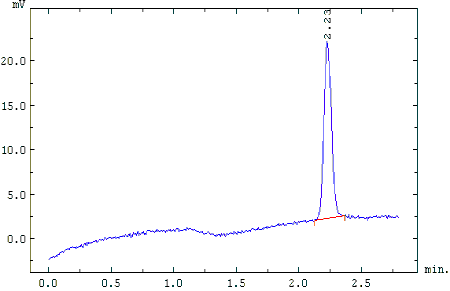
Chromatogram of SF6 extracted from 950cc of water from Delmarva, MD