Natural SF6
Harnisch et al. [1996] and Harnisch and Eisenhauer [1998] reported the presence of SF6 in fluorite, and in two of eight granites analyzed. Busenberg and Plummer [1997] found large concentrations of SF6 in hot springs from volcanic and igneous areas, and low concentrations in pre-1940’s ground waters from Maryland. These results indicated that small concentrations of SF6 were present in waters that pre-dated industrial production of SF6.
The natural background concentration of SF6 as a function of the helium concentration of ground water from the Atlantic Coastal Plain aquifer of Virginia and Maryland, USA. All ground water samples contained no tritium and no CFCs and were older than 60 years. Assuming equilibrium between air and water at recharge, the pre-1940's mixing ratio of SF6 in air was about 0.054 parts per trillion.
SF6 was measured in a suite of minerals and rocks of various origins in an attempt to determine the source of the SF6 (see table below). The detection limit of SF6 using this procedure was 1.7 ± 10-19 mol/g of sample. SF6 was present in almost all the samples that were analyzed in this study because the detection limit was 104 times lower than the detection limit of Harnish and Eisenhauer [1998]. Harnish and Eisenhauer [1998] detected SF6 in nearly all the fluorites they analyzed and in only two of 8 granites.
The highest concentrations of SF6 found in this study were in a fluorite and granite; however, significant concentrations SF6 also were found in other mineral and rock types. SF6 was present in many samples from hydrothermal mineral deposits. The results of this study and the data of Harnish and Eisenhauer [1998] apparently indicate that SF6 concentrations are generally lower in mafic and higher in silicic igneous rocks. Relatively high concentrations of SF6 were present in a sedimentary dolomite and in a Permian salt from the Michigan basin, but no SF6 was detected in a Bermuda calcarenite; suggesting that high concentrations of SF6 may also be present in some diagenetic fluids.
Mineral or rock |
State, province or country | Location | Weight of sample (g) | Volume of gas released at 23°C (cm3 STP/g x100) | Volume of gas released at 250°C (cm3 STP/g x100) | Conc. of SF6 in sample (moles/g x1018) | Conc. of SF6 in gas released at 250°C (pptv) | |
|---|---|---|---|---|---|---|---|---|
|
granite |
Sweden |
Gotemar |
56.5 |
1.11 |
1.19 |
9000 |
19000 |
|
|
well cuttings (granitic) |
New Mexico |
Albuquerque |
37 |
0.075 |
2.19 |
0.92 |
10 |
|
|
microcline |
Ontario |
Bancroft |
49.5 |
1.81 |
2.66 |
12 |
11 |
|
|
quartz diorite** |
Virginia |
Shenandoah Park |
62 |
0.032 |
0.84 |
0.49 |
1.4 |
|
|
diabase |
Virginia |
--- |
59 |
0.003 |
nd |
<0.2 |
bdl |
|
|
scoria |
Idaho |
Big Southern Butte |
26.5 |
npc |
0.009 |
0.65 |
180* |
|
|
labradorite |
New York |
Adironducks |
61.5 |
0.053 |
1.09 |
0.59 |
1.3 |
|
|
fluorite |
Illinois |
Rosiclare |
98.5 |
1.01 |
nd |
7600 |
nd |
|
|
sphalerite-calcite |
Kentucky |
Garrard Co. |
40 |
2 |
nd |
7.9 |
nd |
|
|
Ni-Cu ore + matrix |
Ontario |
Sudbury |
70 |
0.022 |
0.42 |
3 |
17 |
|
|
marble |
New Jersey |
Franklin area |
71.5 |
1.52 |
nd |
27 |
nd |
|
|
marble |
Georgia |
Atlanta |
45 |
0.085 |
nd |
0.25 |
nd |
|
|
mica schist |
Virginia |
--- |
59 |
npc |
0.034 |
<0.2 |
0 |
|
|
dolomite |
Wisconsin |
--- |
65.5 |
0.34 |
1.76 |
10 |
14 |
|
|
halite |
Michigan |
Detroit |
62.5 |
0.021 |
1.18 |
14 |
29 |
|
|
calcarenite |
Bermuda |
--- |
40 |
0.021 |
0.41 |
<0.2 |
bdl |
|
|
detection limit |
--- |
--- |
40 |
0.017 |
--- |
0.17 |
--- |
|
|
nd = not determined |
||||||||
Table showing the concentration of SF6 in various rocks and minerals.
Locust Grove, Maryland
The Locust Grove watershed is located on the Delmarva Peninsula on the Atlantic Coastal Plain. The surficial aquifer is unconfined and consists of sands and gravels, and ranges in thickness from about 25 m at Chesterville Branch in the southern part of the watershed to about 5 m in the northern part. The surficial aquifer is underlain by the Aquia confining layer which consists mainly of silt and clay. Maps showing the geographic location, the location of wells, and the flow paths can be found in Dunkle et al. [1993], Reilly et al. [1994] and Böhlke and Denver [1995].
The area is intensely farmed; principal crops are corn, soybeans, and ornamental shrubs and trees. The ground-water chemistry has been profoundly altered by the agricultural practices [Hamilton and Shedlock, 1992; Hamilton et al., 1993, Böhlke and Denver, 1995]. The concentrations of agricultural chemicals in the ground water can be used to estimate the relative ages of the water at this site [Böhlke and Denver, 1995]. There is good correlation between age and nitrate concentration in the ground water, the results clearly reflect the recent increase in the use of fertilizers.
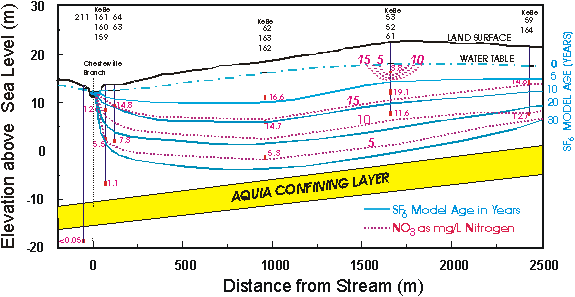
Cross section showing model SF6 ages and nitrate concentrations of the ground water at Locust Grove, Maryland, U.S.
Springs and Ground Water from Igneous and Volcanic Areas
SF6 concentrations greater than that possible for equilibrium with modern air (excess SF6) were detected in discharge from springs throughout the U.S. in areas of igneous and volcanic rocks. A large excess of SF6 was detected in many springs issuing from igneous rocks at or near fault contacts separating crystalline and sedimentary rocks in Maryland, Pennsylvania, Virginia, West Virginia, and New Mexico. Excess SF6 was detected in springs influenced by volcanic activity at Big Springs, Idaho (temperature 13.1°C), located about 12 km west of Yellowstone National Park and 35 km west of Old Faithful Geyser, and Lidy Hot Springs and Condie Hot Spring in southeast Idaho, where water temperatures are 60° and 50°C, respectively.
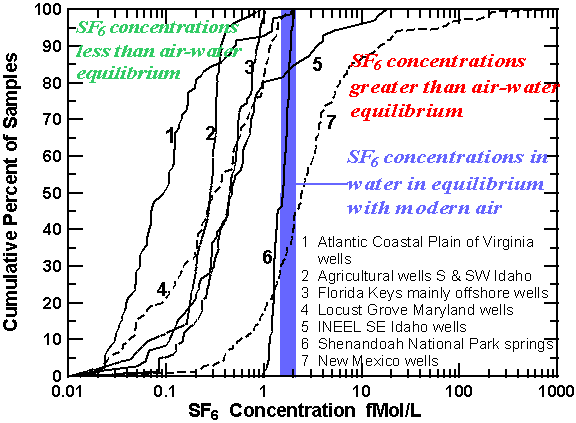
Cumulative percent of concentrations SF6 in fMol/L for ground-water samples collected through out the U.S. Most samples have apparent age, though 20% of the samples from INEEL in Idaho, and 50% of the samples from New Mexico have large excess of SF6
The Chesapeake Bay Watershed of Central-Eastern U.S.
Springs throughout the Chesapeake Bay watershed, an area of approximately 166,000 km2 that drains into the Chesapeake Bay of central-eastern U.S., were sampled for SF6 from 1996 to 1999. The Chesapeake Bay watershed has more than 240,000 km of streams draining six major physiographic provinces. Focazio et al. [1998] give the location of some of the springs, lithology, discharge of springs, major element chemistry, and chlorofluorocarbon concentrations in the waters. Significant differences in SF6 concentrations and ranges of concentration were observed in water of springs from the different physiomorphic and lithologic parts of this watershed.
Spring waters that had the highest specific conductance and the highest dissolved solid content in any given hydrogeomorphic province also had the highest concentrations of SF6. These waters were probably derived from the crystalline rocks and Paleozoic rocks. For example, a sample from Green Spring in the Piedmont crystalline region that contained the highest measured SF6 concentration also had a sulfate concentration of 580 mg/L, which was more than 14 times greater than that in other springs from this region [Focazio et al., 1998]. The unique geochemistry of the spring, older model CFC age of the water, and high SF6 concentration (230 fmol/kg) suggest that a significant fraction of the water was derived from crystalline rocks. In all other springs in this region SF6 concentrations were at or near equilibrium with modern air. The waters of those springs are believed to be mainly derived from regolith, which is composed of saprolite, colluvium, alluvium and soil, because the underlying crystalline rocks have much lower permeability. The high SF6 concentration of Green Spring suggests that the source aquifer contains significant concentrations of SF6. Similar results were found in all other areas with fractured crystalline-rocks. Spring water derived from the overburden, colluvium, saprolite, alluvium or soil had SF6 concentrations that were near or less than air-water equilibrium with modern air.
The springs in the Valley and Ridge carbonate rocks have a greater range of SF6 concentrations than the springs in Piedmont carbonates. About 80 percent of the springs exceeded the modern air-water equilibrium concentration. The Valley and Ridge Carbonates are intensely folded limestones and dolomites of Cambrian to Devonian age and presumably contain high concentrations of SF6. The proximity of the springs to the thrust fault separating the Valley and Ridge Province from the Blue Ridge Mountains may have contributed to the high SF6 concentrations in some of the waters. Springs that are less than 5 km from the thrust fault are represented by filled circles in the next figure. Seven of the eight highest concentrations of SF6 were from springs located near the northwestern side of the Blue Ridge Mountains. Nutter [1974] and Focazio et al. [1998], in their conceptual ground-water-flow models, suggested that a small fraction of the spring water moved through the fractures network of the Blue Ridge mountains, and thus waters may have acquired some of the SF6 from the crystalline rocks.
Spring water from the Coastal Plain is derived from unconsolidated clastic and marine sediments, and concentrations range from less than to near equilibrium between water and modern air. None of the waters had concentrations of SF6 that were significantly higher than modern air-water equilibrium.
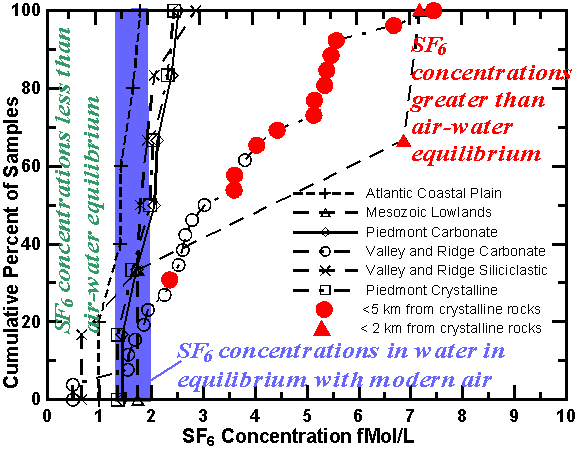
Cumulative percent of concentrations of SF6 in fMol/L for samples collected from springs within the Chesapeake Bay Watershed of eastern U.S. Many of the samples are near equilibrium with modern air or have some apparent age. Filled circles represent samples located within 5 km from a thrust fault separating the Valley and Ridge sedimentary rocks from the Blue Ridge crystalline rocks and are likely enriched from terrestrial sources.