Analytical Procedures for SF5CF3 and CFC-13
The four tracers are determined in the laboratory. The tracers are concentrated on a trap, then released and analyzed by a gas chromatography procedure with an ECD detector. This section describes details of the measurements, including the apparatus for measuring concentrations of the four tracers. The ground waters are equilibrated with a nitrogen headspace in the field, the extracted gases are transferred into 120 ml glass bottles in a 2 liter beaker filled with ground water and capped under water in the field. The procedure for removing the gas samples from the bottles and injecting them into the GC is described below. The measurement procedure, blanks, standardization and calibration procedures, and instrument stability are also given.
Extraction and Measurement of SF5CF3, CFC-13, SF6 and CFC-12
The GC-ECD analytical system was designed to measure concentrations of SF6, CFC-13, SF5CF3 and CFC-12 in gas samples ranging in volume from 6µL to hundreds of mL.
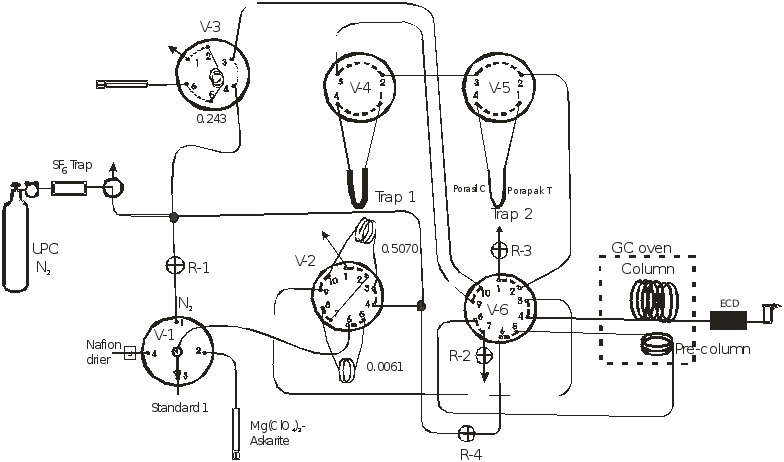
The high concentration segment of the system includes the sample selector valve V-1, and valve V-2 that has two calibrated loops. Standards, blanks, and samples can be injected into the loops though a Nafion® drier or a trap that removes both water and CO2. The sample can be directly injected into the analytical column or the sample can be trapped in trap 2 (V-5) cooled to -70°C in an ethanol-dry ice bath. After trapping, trap 2 is closed and heated to 95°C. The sample then is injected into the pre-column and column by first turning valve V-6 followed by opening the trap. Trap 2 is 15 cm long with diameter of 0.3175 cm, and is packed with 6 cm of Porasil C® and 6 cm of Porapak T® separated with glass wool.
Environmental concentrations of the tracers are injected into the analytical system through selector valve V-3. Air and headspace samples are injected by syringe through a trap that removes both the moisture and CO2. The tracers are trapped on a 1m, 0.3175 cm diameter stainless steel trap packed with Porapak Q® immersed in an ethanol-dry ice bath at -70°C. After trapping the sample in Trap 1, V-3 is turned to position 4 which allows N2 to pass trough the trap at 60 cm3 per minute for two minutes to remove all the trapped O2. Valve V-3 is turned and Trap 2 is opened. Trap 1 then is placed in 95°C water releasing the tracers which then are transferred to the -70°C cooled trap 2. This step transfers the tracers from the larger to the smaller trap in about 1 minute. Trap 2 is closed, placed in 95°C water, V-6 is turned, and then trap 2 is opened, injecting the tracers through the pre-column into the column and the electron capture detector. Both the pre-column and column are of 0.3175 cm diameter stainless steel tubing; all other tubing of the system is 0.158 cm diameter stainless steel. The pre-column is 30cm long and packed with washed 60-80 mesh 5 angstrom molecular sieve (Alltech). The column is 3 m long and packed with 60-80 mesh Carbograph 1AC coated with 1% AT-1000 (Alltech). The ultra pure N2 carrier flow is 30 cm3 per minute. The GC oven is maintained at 65°C and the ECD at 265°C. The analytical time is about 7 minutes per sample.
Calibration of Analytical System
The instrument is calibrated using a blank, and various volumes of a NOAA standard air with known CFC-12 and SF6 concentrations. A gravimetric standard was prepared from pure SF5CF3 and SF6 and diluted with ultra-pure N2. The dilution factor was confirmed by checking the prepared standard with a 1000 pptv Scott Master Gas Standard. The concentrations of the SF5CF3 were calculated in archived air standards and were consistent with the atmospheric mixing ratios of SF5CF3 reported by Sturges et al. [2000]. Since there is no primary CFC-13 standard, an atmospheric concentration of 4 pptv in 1998 clean air [Houghton et al., 2001] was assumed, and all measurements were normalized to this 1998 clean air concentration.
Precision and Accuracy of Measurements
Standard deviations of about 3 percent were routinely obtained for repeated measurements of standards. The calibrations were nearly linear within the measuring range. The analytical precision of the water analyses was about 20 percent near the detection limit of about 0.005 pptv of SF5CF3 in 1970 air, and about 5 percent for ground water recharged after 1985.