CFC Bottle Sampling Method
A procedure that involves filling and capping simple glass bottles with foil-lined caps under water has been tested. Samples analyzed after storage over 6 months demonstrate the validity of the method. This document describes the sampling procedure and presents results of tests with the CFC bottle method.
CFC bottle method
If archival of water samples for CFC or other VOC analysis for periods of more than 6 months is required, then it is recommended that water samples be collected in fused borosilicate ampoules (Busenberg and Plummer, 1992). Otherwise, water samples for CFC analysis can be collected in glass bottles with a foil-lined cap, as described below.
Source of CFC bottles and caps
Order enough bottles and caps to collect four replicates per sample.
The bottles for CFC's are available on USGS OneStop Shopping (internal USGS link). They are stock numbers Q604FLD for the bottles and Q603FLD for the caps.
Bottles and caps can be obtained from Qorpak inc at 800-922-7558. The bottles are 125ml (4 oz) boston round clear glass and have a cap size 22-400. Qorpak item No. 280475 is a case of 24 bottles with the correct aluminum foil lined caps. Qorpak bottle with foil lined cap link
Bottles are also available from any Wheaton glass supplier as Wheaton part number 216802, which is a case of 24 bottles with the wrong caps. Wheaton foil lined caps are wheaton part number 240804 for a case of 1000 caps.
Use only aluminum foil lined caps! The cap is the key to the method. Discard any caps, if the foil liner appears scratched, dented, or altered in any way.
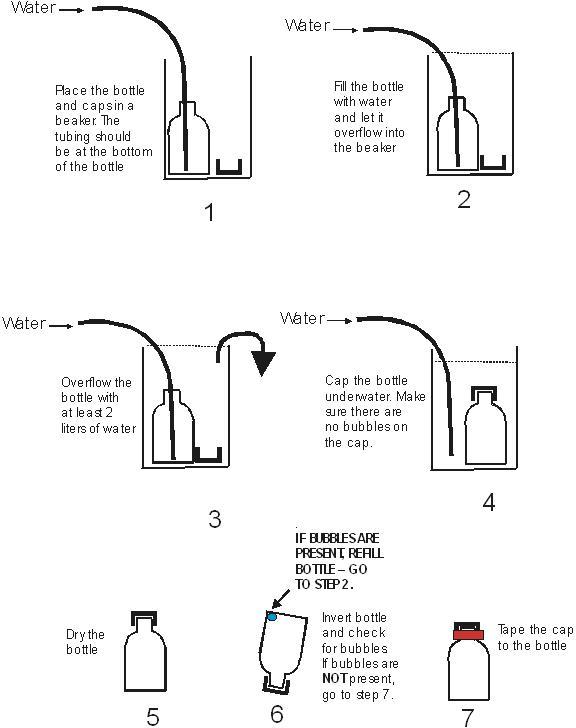
[an error occurred while processing this directive]CFC bottle filling procedure
Instruction given below must be followed to obtain good results with the bottle sampling method for CFCs.
We are receiving too many samples with loose caps and caps that are not properly taped (see below for examples).
The bottles and caps should be thoroughly flushed with the sample water. The bottles are filled under water in a beaker and capped under water. Refrigeration-grade copper tubing is required for CFCs. The filling procedure is carried out within a two to four liter beaker. A plastic beaker is fine. Collect 4 bottles per site.
The procedure is shown below:
- Purge the well according to the USGS National Field Manual.
- A flow rate of between 500ml/min and 2L/min is optimal.
- Place the bottle into a 2-4 liter beaker (plastic acceptable) and then insert the end of the copper tubing from the pump all the way into the bottom of the bottle.
- Fill the bottle with sample water until it overflows.
- Continue to overflow the bottle while it's sitting in the outer beaker until at least one liter of water has flushed through the bottle from the bottom.
- Select a cap and tap it under water to dislodge air bubbles. (the copper tubing inlet line can be used to dislodge bubbles from the cap by pointing it into the cap momentarily while under water)
- Remove the copper tube from the bottle and tightly cap the bottle underwater without allowing the water in the bottle to come in contact with air. Flushing the bottle with more water is far better than with less water.
- Remove the capped bottle from the beaker. RE-tighten the cap. The tighter the cap the better. (pliers are not necessary)
- Invert the bottle, tap it and check it for air bubbles. If there are bubbles, repeat the procedure from step 2 above using a NEW cap.
- If there are no bubbles present, tape the cap securely to the bottle with electrical tape. Wrap the tape in a clockwise direction looking down from the bottle top. Three rounds of electrical tape are needed. The tape helps to keep the cap moist and sealed.
- Label each bottle with the well name, date, and time of sampling and the sequence number of each bottle as it was collected, one through four, in the order of collection.
- Store bottles upside down at room temperature until shipment. The original cardboard box is a good place for storage. The bottles should not be allowed to warm up past room temperature. Storage in a cooler is fine at the field site. Shipment of samples on ice is not necessary. Keep in mind shipping during the summer will require ice to keep the bottles at room temperature (about 23C).
- A bubble will form in most samples while in storage. This is normal.
Examples of properly and improperly sealed CFC bottles

A. Good example. Very tiny bubble formed.
B. Poorly taped cap, air leak - note the large bubble that formed.
C. Cap taped with masking tape, poor seal and large air bubble formed.
Results of tests comparing CFC analyses of waters collected in ampoules and in bottles
A large number of ampoules and bottles were collected from two sources--
(1) water from Hudson Spring which discharges from a limestone karst near the base of the Blue Ridge Mountains at Luray, Virginia, and
(2) water from a deep well in Coastal Plain sands near Milford, Virginia.
Hudson Spring has been sampled for CFCs and 3H/3He over a period of several years and has consistently yielded water with mid-1970s apparent age. Water from the Milford well was expected to be at or near the detection limit for all CFCs. The comparison of ampoules and bottles has continued for 153 days for water from Hudson Spring and 98 days for water from the Milford well. CFC concentrations in water from the Milford well were near or below the detection limit of 2 pg/kg in both ampoules and bottles. In a few cases, water from the Milford well contained detectible CFC-12 but pairs of ampoules and bottles agreed within ± 1 pg/kg in a range of 0 to 10 pg/kg (pre-1954 water). Apparently, there was some small variation in the CFC composition of water discharged from the well. CFC-113 was not detected in either ampoule or bottle, which eliminated the possibility of air contamination during storage. There was an interference of an unknown VOC that gave the appearance of 4-5 pg/kg of CFC-11. Even with the trace interference. The interpreted apparent CFC-11 recharge date was be pre-1950 for CFC-11 which is near the detection limit of the dating method. The figures below compare concentrations of CFC-11, CFC-12, and CFC-113 measured in water from ampoules and bottles from Hudson Spring, as a function of storage time and as a function of collection time. The tests are being continued, but preliminary results indicate that blanks can be collected and stored using the bottle method. It is anticipated that water samples collected in bottles will be analyzed within 4 months of the date they are received at the Reston Chlorofluorocarbon Laboratory. Samples should be shipped promptly to the Reston Chlorofluorocarbon Laboratory following collection.
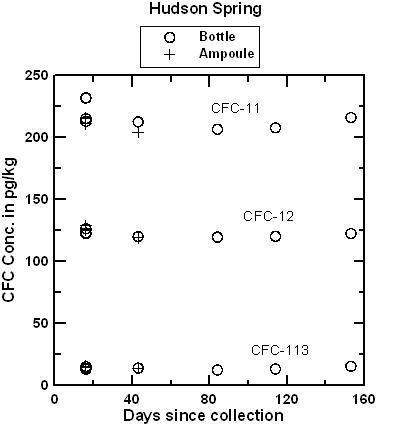
Plot comparing CFC-11, CFC-12 and CFC-113 concentrations in water from Hudson Spring analyzed after storage of more than 40 days in fused borosilicate ampoules and more than 150 days in glass bottles. In the apparent recharge age of the water. The small variations in CFC concentrations are equivalent to differences of less than 0.5 years. And as shown below, the small differences likely reflect differences in concentrations in discharge from the spring over the period of collection of ampoules and bottles (several hours), rather than changes during storage.
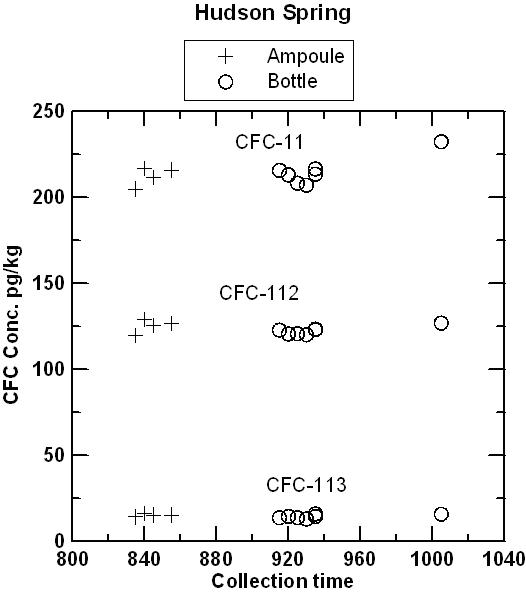
Comparison of CFC-11, CFC-12, and CFC-113 measured in ampoules and bottles plotted in sequence of field collection. The plot suggests that at least some of the very small variations observed represent real variations in water composition discharging from the spring, rather than changes occurring during storage.
CFC sampling photos. Note that outer beaker does not have to be glass. Outer beaker can be glass, metal or plastic.


