3H/3He Dating Background
Tritium (3H, half-life of 12.43 years (Unterweger and others, 1980)) has provided an excellent tracer of young waters. Tritium input to ground water has occurred in a series of spikes following periods of atmospheric testing of nuclear devices that began in 1952 and reached a maximum in 1963-1964. Concentrations of 3H in precipitation have decreased since the mid-1960s bomb peak, except for some small increases from French and Chinese tests in the late 1970s. Radioactive decay of 3H produces the noble gas helium-3 (3He). Tritium measurements alone can be used to locate the depth of the mid-1960s bomb peak, but, because of radioactive decay, many samples may need to be collected and analyzed today to locate its position. In systems younger than the mid-1960s, the bomb peak will not be present due to radioactive decay. Although initial 3H concentrations have decreased because of radioactive decay, measurements of 3H and tritiogenic 3He define a quasi-stable tracer of initial 3H input to ground water and may be used to determine the position of the mid-1960s bomb peak in recharge areas. Additionally, location of the mid-1960s bomb peak provides information on recharge rate (Schlosser and others, 1988, 1989; Solomon and Sudicky, 1991; Solomon and others, 1992, 1993; Ekwurzel and others, 1994).
Locating the position of the mid-1960s bomb peak is difficult due to the required high density of vertical sampling and, therefore, is often an impractical means of obtaining ground-water age information. Determination of the 3H/3He ratio can be used to calculate the 3H/3He apparent age of ground water from a single water sample (Schlosser and others, 1988; 1989; Poreda and others, 1988; Solomon and others, 1992, 1993). The 3H/3He age is based on a helium isotope mass balance used to determine the amount of tritiogenic helium-3 (3Hetri) derived from radioactive decay of 3H in the water sample (Schlosser et al., 1988, 1989). As these substances are virtually inert in ground water, unaffected by ground-water chemistry and unaffected by contamination from most anthropogenic sources, 3H/3He dating can be applied to a wide range of hydrologic investigations. 3H/3He dating complements existing capabilities within the U.S. Geological Survey for dating of young ground water, such as, uses of chlorofluorocarbons and sulfur hexafluoride, and can be applied to dating water recharged since about 1965. Several conditions are necessary to permit solving the helium isotope mass balance for 3Hetri for ground-water samples:
- The sample must contain detectable tritium (greater than approximately 0.5 TU)
- If the sample contains terrigenic He (helium from mantle and crustal sources), Ne data are needed to define 3Hetri,
- The 3He/4He ratio of the terrigenic He, Rterr, must be known
- If the amount of terrigenic He is small (<5 % of the dissolved 4He), the 3H/3He age may be insensitive to even large uncertainties in Rterr
- For samples with a large fraction of terrigenic helium, Rterr (that of mantle and crustal sources) must be known for the particular sample within approximately 1% or better
- If Rterr cannot be defined with sufficient precision to determine age, a range in age can be evaluated for a range in Rterr.
Finally, 3H/3He age and reconstructed initial tritium (3H+3Hetri), after correction for dilution with old (low tritium) water, should be consistent with possible tritium-age relations for surface water or meteoric infiltration water of that age (see for example Figure 14 in Dunkle and others, 1993; Figure 7 in Ekwurzel and others, 1994; and Figure 7 in Plummer and others, 2000).
If the 4He concentration of the water can be attributed solely to atmospheric sources (equilibration with air during recharge and "excess air"), it can be assumed that 3He in the water is of atmospheric and tritiogenic origin. For samples that are not affected by terrigenic helium, the tritiogenic 3He concentration in the water sample is, in this case (Schlosser and others, 1988),
![]()
where 3Hetrit is the tritiogenic 3He in TU, 4HeS is the measured 4He content of the sample in ccSTP/g water, 4Heeq is the 4He content of air-equilibrated water at the recharge temperature in ccSTP/g water, RS is the measured 3He/4He ratio of the sample, Ra,is the 3He/4He ratio of air (1.384x10-6, Clarke and others, 1976), and a is the equilibrium isotope fractionation factor (0.983, Benson and Krause, 1980). The constant 4.021·1014 converts the unit ccSTP/g water to TU. [One TU is equal to 1 3H atom in 1018 atoms of H, or 3.24 picocuries per liter, pCi/L. One liter of water with a concentration of 1 TU produces 7.2 disintegrations per minute(dpm) or 0.12 becquerel (Bq); one Bq corresponds to 1 disintegration per second (dps);one curie (Ci) is equal to 3.7x1010 Bq.]
Additional He sources, terrigenic He, may be present in aquifers where the rocks are enriched in U or Th, or in ground-water samples in which young water has mixed with relatively old water containing terrigenic He. In these cases, the measured Ne content (assumed to be derived solely from the atmosphere) can be used to calculate the additional He (Heterr.; Schlosser and others, 1989)--
![]()
where 4Heterr. is the terrigenic 4He concentration, Nes is the measured neon concentration in the sample, Neeq is the Ne concentration in water in equilibrium with air, and (4He/Ne)atm is the atmospheric ratio (0.288). The 3Hetrit then becomes
![]()
where Rterr. is the 3He/4He ratio of the terrigenic He source.
Rter has to be determined from the isotope measurements of tritium-free water in the aquifer under investigation. For dating studies of waters from crystalline rocks, or even for waters from alluvial aquifers associated with crystalline bedrock, it is best to sample several old (tritium-free) waters to aid in defining Rterr. and interpretation of age. If the 3Hetrit is confined in the aquifer, the apparent 3H/3He age, t (in years) of the water can be calculated as follows (Schlosser and others, 1988):
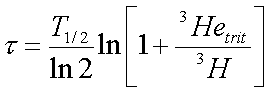
where T1/2 is the 3H half-life.
Schlosser and others (1988, 1989) reported 3H/3He dating of shallow ground water sampled from wells screened at multiple levels at Liedern/Bocholt, Germany. The 3H from 1963-64 atmospheric nuclear-bomb testing was clearly evident in the tritiogenic 3He at a depth of 5 to 10 meters in the saturated zone. 3H/3He ages of the bomb-pulse waters were 3 to 5 years younger than the true age (1963). This difference was attributed to incomplete 3He confinement and dispersive mixing with deeper water. From estimates of the 3H infiltration, Schlosser and others (1988) estimated that approximately 80 percent of the tritiogenic 3He remained in the ground water at Liedern/ Bocholt. Calculations based on the "Vogel" model (Vogel 1967), as applied to shallow, homogeneous sand aquifers of isotropic hydraulic conductivity, showed that the shape of the bomb pulse will be detectable in tritiogenic 3He data for at least the next 40 years, long after the bomb pulse is lost in the 3H data because of radioactive decay and advection/dispersion (Schlosser and others 1989).
Helium-3 confinement has also been shown to be a function of the vertical flow velocity (recharge rate) and dispersivity. Schlosser and others (1989) calculated significant 3He loss across the water table to the atmosphere at vertical flow velocities of less than 0.25 to 0.5 m/yr. Although absolute 3H/3He ages are less certain when recharge rates are small, location of the position of the bomb pulse, expressed in tritiogenic 3He, is of great value in hydrologic studies and can be used to determine ground-water velocities.
Solomon and Sudicky (1991, 1992) used numerical simulations of simple one- and two-dimensional flow systems in hypothetical unconfined, shallow sandy aquifers to investigate the sensitivity of calculated 3H/3He ages to hydrodynamic dispersion. These authors showed that the magnitude of uncertainties in calculated 3H/3He ages depends on the 3H input. When 3H input is nearly constant over time, such as the 3H input in recharge since the mid- to late 1970s, calculated 3H/3He ages tend to be within 10 percent of true ages. However, under transient conditions, such as for waters recharged prior to the 1960s bomb pulse, dispersion can cause more than 50 percent differences between calculated 3H/3He ages and advective travel times. If the vertical velocity is rapid enough to maximize 3He confinement (Schlosser and others 1989), 3H/3He ages determined near the water table should closely reflect the average vertical velocity.
Uncertainty in age because of analytic uncertainty is approximately ± 0.5 years. Larger uncertainties in age result from corrections in defining the tritiogenic 3He, the requirement that the parcel of water remain confined following infiltration, and mixing effects caused by hydrodynamic dispersion. If 3He is lost by diffusion to the unsaturated zone air, younger ages are derived. 3He can also be added to shallow ground water by dispersive transport. Due to the variable nature of the 3H input, 3H/3He dating becomes less certain for waters older than the mid-1960s bomb pulse due to dispersive mixing. Consequently, 3H/3He dating is most reliable only for post-1970 water (Solomon and others 1992) when 3H input has been relatively constant and therefore influenced to a lesser extent by hydrodynamic dispersion (Solomon and Sudicky 1991). See research for more information.
Groundwater Mixtures--CFCs vs 3H/3He
Practical applications of environmental tracers to dating young ground water often depend on sampling from pre-existing domestic, industrial, and municipal-supply wells that, because of their construction, intercept relatively large open intervals and can produce mixed waters. The age of the young fraction(s) in ground-water mixtures can be particularly useful when assessing the susceptibility of ground-water resources to contamination from anthropogenic sources. There are fundamental differences between dating the young fraction in ground-water mixtures with CFCs and with 3H/3He. In the case of simple binary mixtures of old (recharged before about 1940) water and young water, the source of CFCs and 3H can be almost entirely attributed to the young fraction. In some mixtures, the CFC age of the young fraction could be determined from the ratio of two CFCs in the water sample, and mixing fractions based on the ratio of observed to expected CFC concentrations in the water sample (see Plummer and others, 2000).
If mixing of young and old water occurs, the CFC concentration in the ground-water sample must be divided by the fraction of young water in the mixture before age of the young fraction can be estimated. The CFC age of the young fraction is then computed by comparing air concentrations that would be in equilibrium with the CFC concentration in the young fraction with historical air concentrations (Busenberg and Plummer, 1992). If the mixture contains multiple fractions of young water, the resulting age is regarded as a mean age of the young fraction(s) in the mixture.
Effects of mixing can be more significant when waters are sampled from relatively large intervals in aquifers. Mixing, if it occurs, may not be readily apparent if the concentration of a particular constituent (such as dissolved Cl- or Ca2+) in the aquifer is uniform over the depth interval sampled. When sampling young ground water or mixtures containing young ground water for transient tracers such as CFCs and 3H/3He, there will almost always be compositional gradients, especially when ground water is sampled from relatively large intervals in aquifers.
In any mixture containing a fraction of post-bomb water and a fraction of pre-bomb water, the detectable 3H and 3Hetrit is attributed to the young water fraction. Compared to 3H and 3He derived from the young water fraction, any contribution of 3H and 3He from the old water fraction is, to a good approximation, negligible and the calculated 3H/3He age applies to the post-bomb fraction of the mixture. If mixtures of more than one post-bomb water fraction of different age occur, the calculated 3H/3He age will be intermediate to the ages of the post-bomb fractions. There is usually insufficient data for resolving mixtures of more than one post-bomb water in ground-water mixtures, and consequently, the reported ages (both 3H/3He- and CFC-based ages) should be regarded as mixed ages for the young fraction(s) in each sample. See for example Plummer and others (1998a, 1998b, 2000).
References Cited
Bayer, R., Schlosser, P., Bonisch, G., Rupp, H., Zaucker, F., and Zimmek, G., 1989, Performance and blank components of a mass spectrometric system for routine measurement of helium isotopes and tritium by the 3He ingrowth method: Sitzungsberichte der Heidelberger Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse, Jahrgang 1989, 5. Abhandlung, Springer-Verlag, 42pp.
Benson, B.B., and Krause, D., Jr., 1980, Isotopic fractionation of helium during solution: A probe for the liquid state: J.Soln.Chem., v. 9, p. 895-909.
Busenberg, E., and Plummer, L.N., 1992, Use of chlorofluoromethanes (CCl3F and CCl2F2) as hydrologic tracers and age-dating tools: Example - The alluvium and terrace system of central Oklahoma: Water Resources Research, v. 28, p. 2257-2283.
Busenberg, E., Weeks, E.P., Plummer, L.N., and Bartholemay, R.C., 1993, Age dating ground water by use of chlorofluorocarbons (CCl3F and CCl2F2), and distribution of chlorofluorocarbons in the unsaturated zone, Snake River Plain aquifer, Idaho National Engineering Laboratory, Idaho: U.S. Geological Survey Water-Resources Investigations Report 93-4054, 47p.
Clark, W.B., Jenkins, W.J., and Top, Z., 1976, Determination of tritium by mass spectrometric measurement of 3He: International Journal of Applied Radiation and Isotopes, v. 27, p. 515-522.
Cook, P.G., Solomon, D.K., Plummer, L.N., Busenberg, E., and Schiff, S.L., 1995, Chlorofluorocarbons as tracers of groundwater transport processes in a shallow, silty sand aquifer: Water Resources Research, v. 31 no. 3, p. 425-434.
Dunkle, S.A., Plummer, L.N., Busenberg, E., Phillips, P.J., Denver, J.M., Hamilton, P.A., Michel, R.L., and Coplen, T.B., 1993, Chlorofluorocarbons (CCl3F and CCl2F2) as Dating Tools and Hydrologic Tracers in Shallow Ground Water of the Delmarva Peninsula, Atlantic Coastal Plain, United States: Water Resources Research, v. 29, no. 12, p. 3837-3860.
Ekwurzel, B., Schlosser, P., Smethie, Jr., Plummer, L.N., Busenberg, E., Michel, R.L., Weppernig, R., and, Stute, M., 1994, Dating of shallow groundwater: Comparison of the transient tracers 3H/3He, chlorofluorocarbons and 85Kr: Water Resources Research, v. 30, no. 6, p. 1693-1708.
Elkins, J. W.; Thompson, T. M.; Swanson, T. H.; Butler, J. H.; Hall, B. D.; Cummings, S. O.; Fisher, D. A.; Roffo, A. G., 1993, Decrease in the growth rates of atmospheric chlorofluorocarbons 11 and 12: Nature, v. 364, p. 780-783.
Katz, B.G., Lee, T.M., Plummer, L.N., and Busenberg, E., 1995, Chemical Evolution of groundwater near a sinkhole lake, northern Florida. 1. Flow patterns, age of groundwater, and influence of lakewater leakage: Water Resources Research, v. 31, no. 6, p. 1549-1564.
Plummer, L.N., Michel, R.L., Thurman, E.M., and Glynn, P.D., 1993, Environmental tracers for age-dating young ground water: in Alley, W.M., ed., Regional Ground-water Quality, Chap. 11, Van Nostrand Reinhold, New York, p. 255-294.
Plummer, L.N., McConnell, J.B., Busenberg, E., Drenkard, S., Schlosser, P., and Michel, R.L., 1998a, Flow of river water into a karstic limestone aquifer-1. Tracing the young fraction in groundwater mixtures in the Upper Floridan aquifer near Valdosta, Georgia. Applied Geochemistry, v. 13(8), p. 995-1015.
Plummer, L.N., Busenberg, E., Drenkard, S., Schlosser, P., McConnell, J.B., Michel, R.L., Ekwurzel, B., and Weppernig, R., 1998b, Flow of river water into a karstic limestone aquifer-2. Dating the young fraction in groundwater mixtures in the Upper Floridan aquifer near Valdosta, Georgia. Applied Geochemistry, v. 13(8), p. 1017-1043.
Plummer, L.N., Rupert, M.G., Busenberg, E., and Schlosser, P., 2000, Age of irrigation water in ground water from the Eastern Snake River Plain aquifer, south-central Idaho. Ground Water, 38(2), 264-283.
Poreda, R.J., Cerling, T.E., and Solomon, D.K., 1988, Tritium and helium isotopes as hydrologic tracers in a shallow unconfined aquifer: Journal of Hydrology, v. 103, p. 1-9.
Reilly, T.E., Plummer, L.N., Phillips, P.J., and Busenberg, E., 1994, The use of simulation and multiple environmental tracers to quantify groundwater flow in a shallow aquifer: Water Resources Research, v. 30, no. 2, p. 421-433.
Schlosser P., Stute, M., Dorr, H., Sonntag, C., and Munnich, K.O., 1988, Tritium/3He dating of shallow groundwater: Earth and Planetary Science Letters, v. 89, p. 353-362.
Schlosser P., Stute, M., Sonntag, C., and Munnich, K.O., 1989, Tritiogenic 3He in shallow groundwater: Earth and Planetary Science Letters, v. 94, p. 245-256.
Solomon, D.K., and Sudicky, E.A., 1991, Tritium and helium 3 isotope ratios for direct estimation of spatial variations in groundwater recharge: Water Resources Research, v. 27, no. 9, p. 2309-2319.
Solomon, D.K., Poreda, R.J., Schiff, S.L., and Cherry, J.A., 1992, Tritium and Helium 3 as groundwater age tracers in the Borden Aquifer: Water Resources Research, v. 28, no. 3, p. 741-755.
Solomon, D.K., and Sudicky, E.A., 1992, Correction to "Tritium and helium 3 isotope ratios for direct estimation of spatial variations in groundwater recharge": Water Resources Research, v. 28, no. 4, p. 1197.
Solomon, D.K., Schiff, S.L., Poreda, R.J., and Clarke, W.B., 1993, A validation of the 3H/3He method for determining groundwater recharge: Water Resources Res., v. 29, no. 9, p. 2951-2962.
Szabo, Z., Rice, D.E., Plummer, L.N., Busenberg, E., Drenkard, S., and Schlosser, P., 1996, Age dating of shallow groundwater with chlorofluorocarbons, tritium helium 3, and flow path analysis, southern New Jersey coastal plain: Water Resources Research, v. 32, no. 4, p. 1023-1038.
Thompson, G.M, 1976, Trichloromethane, a new hydrologic tool for tracing and dating ground water: Bloomington, Indiana, Ph.D. Dissertation, Department of Geology, Indiana University. Thompson, G.M., and Hayes, J.M., 1979, Trichloromethane in groundwater - A possible tracer and indicator of groundwater age: Water Resources Research, v. 15, p. 546-554.
Unterweger, M.P., Coursey, B.M., Schima, F.J., and Mann, W.B., 1980, Preparation and calibration of the 1978 National Bureau of Standards tritiated-water standards: Internat. J. Appl. Rad. Isotope, v. 31, p. 611- 614.
Vogel, J.C., 1967, Investigation of groundwater flow with radiocarbon: In Isotopes in Hydrology, Proceedings, IAEA, Vienna, 1967, p. 355-369.