 Stream Water Quality in the Conterminous United States --
Status and Trends of Selected Indicators During the 1980's
Stream Water Quality in the Conterminous United States --
Status and Trends of Selected Indicators During the 1980's
 Stream Water Quality in the Conterminous United States --
Status and Trends of Selected Indicators During the 1980's
Stream Water Quality in the Conterminous United States --
Status and Trends of Selected Indicators During the 1980's
Go to Table of Contents
or Go to previous section
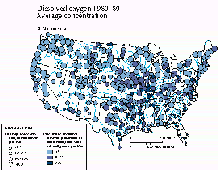
A. Map of the conterminous United States showing average concentration and the percentage of samples (frequency of occurrence) at each water-quality station in which concentrations were greater than or less than the selected concentration thresholds at each station (figs. 38A-43A). These thresholds represent either EPA recommended concentration limits (U.S. Environmental Protection Agency, 1976, 1986) or other arbitrarily defined thresholds chosen to separate high and low concentrations.
B. Box plot showing average station concentrations of the constituent, by land use (figs. 38B-43B).
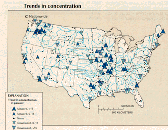
C. Map of the conterminous United States showing the trend in concentration, which was derived from applying the seasonal Kendall test to the constituent-concentration records at each station (figs. 38C-43C). For stations with sufficient concentration and streamflow data, concentration records were flow adjusted before trend testing. About 86 percent of the stations were flow adjusted. (See article "Statistical Analysis of Water- Quality Data" in this volume for more discussion of the seasonal Kendall test.)
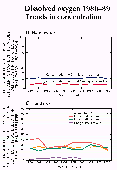
D. Graph showing the percentage of stations where the annual average concentration was greater than or less than selected concentration thresholds (figs. 38D-43D).
E. Graph showing the percentage of the stations where the annual average concentration was greater than or less than selected concentration thresholds, by land use (figs. 38E-43E).
In the absence of substances that cause its depletion, the dissolved-oxygen concentration in stream water approximates the saturation level for oxygen in water in contact with the atmosphere and decreases with increasing water temperature from about 14 mg/L (milligrams per liter) at freezing to about 7 mg/L at 86 degrees F (30 degrees C). For this reason, in ecologically healthy streams, the dissolved-oxygen concentration depends primarily on temperature, which varies with season and climate.
Criteria for defining desirable dissolved-oxygen concentration often are differentiated as applicable to cold- water biota, such as trout and their insect prey, and the more low-oxygen-tolerant species of warm-water ecosystems. Moreover, because of the critical respiratory function of dissolved oxygen in aquatic animals, criteria often are expressed in terms of the short-term duration and frequency of occurrence of minimum concentrations rather than long-term average concentrations. Studies cited by the EPA (1986) of the dependence of freshwater biota on dissolved oxygen suggest that streams in which the concentration is less than 6.5 mg/L for more than about 20 percent of the time generally are not capable of supporting trout or other cold-water fish, and such concentrations could impair population growth among some warm-water game fish, such as largemouth bass. Furthermore, streams in which the dissolved-oxygen-deficit concentration is greater than 4 mg/L for more than 20 percent of the time generally cannot support either cold- or warm-water game fish. Dissolved-oxygen deficit refers to the difference between the saturation and measured concentrations of dissolved oxygen in a water sample and is a direct measure of the effects of oxygen-demanding substances on dissolved oxygen in streams.
Major sources of substances that cause depletion of dissolved oxygen in streams are discharges from municipal and industrial wastewater-treatment facilities; leaks and overflows from sewage lines and septic tanks; stormwater runoff from agricultural and urban land; and decaying vegetation, including aquatic plants from the stream itself and detrital terrestrial vegetation. Dissolved oxygen is added to stream water by the process of aeration (waterfalls, ripples) and the photosynthesis of aquatic plants.
Figure 38A shows the average concentration of dissolved oxygen and the percentage of samples (frequency of occurrence) of concentrations less than 6.5 mg/L at each of 424 selected stations. One readily apparent feature of the map is a climate-related pattern of decreasing average dissolved-oxygen concentration from north to south, which reflects the greater capacity of colder water to contain dissolved oxygen. The lowest average concentrations (less than 8.0 mg/L) and the highest frequencies of concentrations less than 6.5 mg/L occurred in the Southeast where high temperatures and a larger than average burden of decaying vegetation are natural constraints on dissolved-oxygen concentration. Disrupting this pattern, however, are a few stations in the northern tier of States and numerous stations in the central States at which the average concentrations were less than 8.0 mg/L and where concentrations less than 6.5 mg/L occurred for more than 20 percent of the samples. Among the four land-use types, the average concentrations of dissolved oxygen were the lowest at stations in urban areas (fig. 38B).
 A.
A.
 B.
B.
 D.,E.
D.,E.
Upward trends (38 stations) in dissolved-oxygen concentration outnumbered downward trends (26 stations), as shown in figure 38C. In general, increases in dissolved oxygen represent an improvement in water quality. Concentration increases were especially numerous in the central States where at many stations concentration levels were less than the 6.5 mg/L criterion for more than 20 percent of the samples. Nationally, the percentage of stations having more than 20 percent of concentrations less than 6.5 mg/L remained nearly constant at about 20 percent. Similarly, the percentage of stations having more than 20 percent of dissolved-oxygen-deficit concentrations greater than 4.0 mg/L remained constant at about 9 percent (fig. 38D). In urban areas, however, the percentage of stations having more than 20 percent of dissolved-oxygen concentrations less than 6.5 mg/L decreased from 42 in 1981 to 21 percent in 1989 (fig. 38E).
Changes in dissolved-oxygen concentrations in the Nation's streams during the 1980's are of particular interest given the large public and private capital investments made to control point-source pollution during the decade. It is estimated that between 1980 and 1989, $126 billion was spent to upgrade municipal treatment facilities, and $68 billion was spent by private industry to reduce point-source biochemical-oxygen-demand loads (U. S. Environmental Protection Agency, 1990c). Increased dissolved-oxygen concentration in streams in urban areas may reflect investments in point-source pollution control.
At the national level, however, part of the explanation for the lack of change in dissolved-oxygen concentration could be that much of the investment in point-source pollution controls simply has served to keep pace with population increases and economic development. Oxygen-demanding waste loads declined substantially in the 1970's but were nearly stable during the 1980's (U. S. Environmental Protection Agency, 1990c). Despite point-source control expenditures, the loads did not decline in the 1980's, because during that decade the population increased by 10 percent and the real gross national product increased by 30 percent (U.S. Bureau of the Census, 1990). The maintenance of nearly constant dissolved-oxygen concentration in streams during this period of increased pollution generation represents an important environmental benefit of pollution controls.
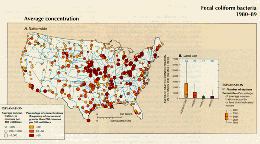
Figure 39 indicates that concentrations of fecal coliform bacteria greater than 200 colonies/100 mL were widespread and frequent in the Nation's streams during the 1980's, but that gradual progress was made in reducing bacterial concentrations over the course of the decade. Fecal-coliform concentrations of 200 colonies/100 mL or greater occurred in at least 20 percent of the samples at a significant majority of the 313 stations shown in figure 39A. Fecal-coliform concentrations were highest in the agricultural areas of the midwestern and south-central parts of the country and in several tributaries to the eastern Great Lakes. At many stations in the agricultural areas, average bacterial concentrations were greater than 1,000 colonies /100 mL. In agricultural and urban areas, average concentrations of fecal coliform bacteria were greater than those in forested and range areas (fig 39B); this is expected because the former areas are known to contain point and nonpoint sources of fecal bacteria.
 A.,B.
A.,B.
 C.,D.,E.
C.,D.,E.
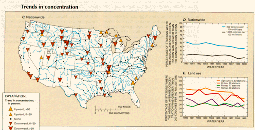
Downward trends in fecal-coliform concentrations (fig. 39C) occurred at 40 stations and upward trends at 10 stations. Concentration decreases were especially common in the central part of the country, and they occurred in cataloging units that had significant urban, agricultural, or range land use. The percentage of stations nationwide where the annual average concentration was greater than 1,000 colonies/100 mL decreased from 18 to 13 percent, and the percentage of stations having annual average concentrations greater than 200 colonies/100 mL decreased from 52 to 35 percent (fig. 39D.) From 1980 to 1989, in all land-use areas except forested areas, the percentage of stations in which the annual average concentration was greater than 200 colonies/100 mL decreased (fig. 39E). All of these trends suggest that control of point and nonpoint sources of fecal coliform bacteria improved over the course of the decade.
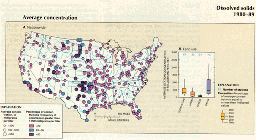
In most streams the major source of dissolved solids is the dissolution of minerals naturally found in soil and rock. (See article "Factors Affecting Stream Water Quality, and Water-Quality Trends in Four Drainage Basins in the Conterminous United States, 1905-90" in this volume for discussion of mineral dissolution.) Because of the wide variation in the solubility of different minerals, and in the amount of precipitation available to dissolve them, the concentration of dissolved solids in streams nationwide ranges from only a few milligrams per liter to several thousand milligrams per liter. Within this broad range, the highest dissolved-solids concentrations (greater than 500 mg/L) are found in the arid Southwest, where high rates of evaporation and transpiration tend to concentrate dissolved solids. Concentrations are medium to high (greater than 100 mg/L) in parts of the midwestern States, where soluble carbonates are abundant, and they are lowest (less than 100 mg/L) in the eastern and northwestern parts of the United States, where high precipitation rates dilute dissolved constituents (fig. 40A). Average concentrations were lowest in forested areas and highest in range areas, which reflect the more arid areas of the country (fig. 40B).
 A.,B.
A.,B.
 C.,D.,E.
C.,D.,E.
Human activities contribute significantly to dissolved-solids concentrations in most regions, however. A moderate correlation between population and dissolved-solids concentration in streams has long been noted (Peters, 1984) for much of the eastern and northwestern United States, where point-source municipal and industrial effluents typically have higher dissolved-solids concentrations than their receiving streams. Also, land disturbance associated with mining and agriculture increases the exposure of mineral deposits to precipitation and increases the nonpoint-source load of dissolved solids. In recent years, the correlation between population and dissolved-solids concentrations has been strengthened by the increased dissolved salts in streams as a result of the increased use of highway deicing salt (Smith and others, 1987). In addition, in arid regions, reservoirs have increased evaporation rates, which result in higher stream concentrations of dissolved solids (Paulson and Bakers, 1983).
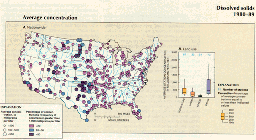
Nationwide trends in dissolved-solids concentration varied throughout the 1980-89 period, and downward trends (46 stations) outnumbered upward trends (28 stations) among a total of 340 stations (fig. 40C). Downward trends were especially common in the central part of the country, the Pacific Northwest, and far southwestern United States, whereas upward trends were most common in drainage to the Gulf of Mexico and Atlantic Ocean. Nationally, the percentage of stations having average concentration greater than 100, 500, and 1,000 mg/L remained approximately constant at 75, 25, and 12 percent, respectively (fig. 40D). Moreover, among specific land-use classes, the percentage of stations exceeding the 500 mg/L threshold remained nearly constant (fig. 40E).
Ecological concern about high concentrations of nitrate in streams stems from its potential for contributing to eutrophication, which is the excessive growth of aquatic plants that can impart unpleasant odors and tastes to water and reduce its clarity, and, upon dying, can lower the dissolved-oxygen concentrations (National Academy of Sciences, 1969). It has not been possible, however, to establish a nationally applicable threshold concentration for nitrate to protect against eutrophication because effects of nitrate concentrations are highly variable from place to place and are greatest in coastal waters that are far removed from inland nitrate sources. Historically, government standards and eutrophication-control strategies for inland waters have focused on phosphorus concentration rather than on nitrate concentration because phosphorus usually is depleted more rapidly by the growth of aquatic plants than is nitrate, and, therefore, frequently is the limiting factor in eutrophication. Increasingly, however, it is recognized that control of estuarine and coastal eutrophication will require control of nitrate from inland sources.
Major sources of nitrate in streams are municipal and industrial wastewater discharge and agricultural and urban runoff. Deposition from the atmosphere of the nitrogenous material in automobile exhaust and industrial emissions also is a source.
For purposes of this article, nitrate concentrations are arbitrarily grouped into three concentration classes--less than 1 mg/L, 1 to 3 mg/L, and greater than 3 mg/L. Figure 41A shows that average nitrate concentrations were greater than 3 mg/L at several stations in the midwestern and southwestern United States. The concentrations ranged from 1 to 3 mg/L at many other stations in those areas, as well as in the mid-Atlantic States and the lower Mississippi River basin. Throughout much of the rest of the Nation, nitrate concentrations averaged less than 1 mg/L. At stations in agricultural and urban areas, average concentrations of nitrate were much greater than concentrations at stations in forested and range areas (fig. 41B).
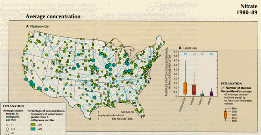 A.,B.
A.,B.
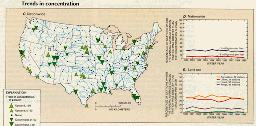 C.,D.,E.
C.,D.,E.
Significant trends in nitrate concentration (fig. 41C) were nearly equally divided between upward (22 stations) and downward (27 stations) trends among a total of 344 stations. Downward trends occurred predominately in the eastern, south-central, and southeastern United States, whereas upward trends were geographically scattered. Nationally, the percentage of stations having average concentrations greater than 1 mg/L remained constant at about 20 percent (fig. 41D). There is some evidence of success in reducing nitrate levels in streams having very high concentrations because the percentage of stations nationwide having annual average concentrations greater than 3 mg/L decreased from about 6.5 to 4.0 percent. In agricultural areas, the percentage of stations where the annual average concentration was greater than 1 mg/L reached a peak of 46 percent in 1984 and then declined to 34 percent by 1989 (fig. 41E).
These 1980-89 trends in nitrate concentration represent a noteworthy change from the period 1974-81, when increases in nitrate were widespread and appeared related to large increases in nitrogen-fertilizer use through 1981 (Smith and others, 1987). The lack of a nationwide trend in nitrate concentration in streams during the 1980's, therefore, is consistent with the fact that nitrogen fertilizer use peaked in 1981 and has remained approximately at that level since (Alexander and Smith, 1990).
Phosphorus is a particularly important nutrient in freshwater ecosystems because, as discussed above in the nitrate section, phosphorus usually is the nutrient in shortest supply and its availability often controls the rate of eutrophication. When human activities make phosphorus available in larger quantities, the accelerated growth of algae and other aquatic plants in streams can cause eutrophication, which depletes dissolved oxygen, imparts undesirable tastes and odors in the water, and clogs water-supply intakes. To protect against eutrophication, the EPA (1986) recommends an upper limit of 0.1 mg/L as the standard for total phosphorus in streams. For this article, a threshold of 0.1 mg/L and an arbitrary threshold of 0.5 mg/L were selected for analysis of total- phosphorus concentrations in streams. Several characteristics of eutrophication in lakes and reservoirs, such as algal biomass, water clarity, and dissolved-oxygen depletion rate, have been found to be strongly correlated with the loading rate of total phosphorus (Rast and others, 1983). Estimates of phosphorus loading for a national sample of reservoirs are discussed in the section "Transport to Selected Reservoirs."
Sources of phosphorus are the decomposition of organic matter and inorganic phosphate minerals that are mined and incorporated in fertilizers, detergents, and other commodities. Thus, major point sources of phosphorus to streams are waste discharges from sewage-treatment and food-processing plants and other industrial facilities. Nonpoint sources of phosphorus include agricultural and urban runoff and, in certain regions, the runoff and ground-water flow from areas that contain natural deposits of phosphate minerals (Hem, 1985).
As discussed in the article "Introduction to State Summaries of Stream Water Quality" in this volume, apparent bias in the analysis of total phosphorus by the USGS in water years 1980 and 1981 made analysis of trends in total phosphorus unsuitable for those years. Consequently, all analyses for total phosphorus in this article are reported for water years 1982-89, a period for which no measurement bias is known to exist.
Average total-phosphorus concentrations were 0.1 mg/L or greater at most of the 410 stations shown in fig. 42A. Concentrations greater than 0.5 mg/L were especially common at stations in the central and south- central part of the country where extensive agricultural use of phosphorus and highly erodible soils combine to create large nonpoint-source loads of phosphorus. Average total-phosphorus concentrations for the 1982-89 period show a higher concentration in agricultural areas but show a wider range of concentrations in urban areas (fig. 42B). Total-phosphorus concentrations are high in urban areas, where streams are influenced by a combination of point and nonpoint sources, and in west-central Florida, where there are large deposits of phosphate minerals.
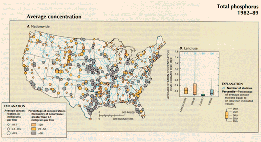 A.,B.
A.,B.
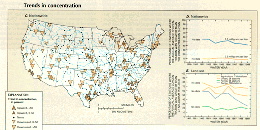 C.,D.,E.
C.,D.,E.
Nationally, downward trends (92 stations) in total phosphorus (fig. 42C) occurred very widely and outnumbered upward trends (19 stations) by a factor of almost 5 to 1. Downward trends occurred in all regions but occurred most frequently in the central States and the Great Lakes region. Upward trends occurred most frequently in the southeastern States. Nationally, the percentage of stations having annual average total-phosphorus concentrations greater than 0.1 mg/L decreased gradually from 54 to 42 percent between 1982 and 1989 (fig. 42D). A decrease in the percentage of water-quality monitoring stations having annual average concentrations greater than 0.1 mg/L occurred in all land-use categories with the exception of heavily forested areas, where the concentrations were more constant (fig. 42E).
The widespread pattern of a decline in total-phosphorus concentrations during the 1980's appears to represent the geographic expansion of a pattern begun in the 1970's (Smith and others, 1987) when downward trends were only slightly more numerous than upward trends, and occurred mostly in the Great Lakes area and the upper Mississippi River basin. Upward trends occurred most frequently in the Southeast. It is likely that widespread declines in total-phosphorus concentrations during the 1980's reflect significant reductions in point- source loads that occurred during the period and some reduction in nonpoint-source loads. In addition to the previously described improvements in municipal- and industrial-wastewater treatment, the decrease since 1971 in phosphorus content of detergents helped reduce point-source loads by an estimated 15 to 20 percent (U.S. Environmental Protection Agency, 1990b). The reasons for changes in nonpoint-source loads of phosphorus are more difficult to determine, but phosphorus-fertilizer use has declined more than 20 percent since 1979 (Alexander and Smith, 1990), and phosphorus contributions from livestock waste declined about 10 percent from 1982 to 1987 (U.S. Bureau of the Census, 1989; U.S. Soil Conservation Service, 1992).
The source of most suspended sediment is soil erosion. Although organic particles frequently form an important component of suspended sediment, most is inorganic by weight. Rates of soil erosion vary widely and depend on such factors as soil characteristics, precipitation frequency and intensity, slope of the land surface, and the nature and extent of land disturbance from agriculture, mining, and construction. Because the quantities of sediment entering streams depend greatly on natural factors, it is difficult to establish national criteria for suspended-sediment concentration. In many western areas, for example, stream ecosystems are naturally adapted to suspended-sediment concentrations that periodically are many times greater than those that are detrimental in other areas. Rather than establish national criteria for suspended-sediment concentration, the EPA has recommended that light penetration in water not be reduced by suspended material by more than 10 percent from its natural level (U.S. Environmental Protection Agency, 1986). In this article, average suspended-sediment concentrations for this study period are arbitrarily grouped into three concentration classes--less than 100 mg/L, 100 to 500 mg/L, and greater than 500 mg/L.
Suspended-sediment concentrations at the 324 stations shown in figure 43A were highest in the west- central part of the country, where many water-quality monitoring stations had average concentrations greater than 500 mg/L and some had concentrations greater than 1,000 mg/L. By contrast, average concentrations only rarely were 100 mg/L or greater at stations in the north Atlantic and south Atlantic States, Great Lakes, and Pacific Northwest. Throughout much of the central part of the United States, average concentrations were in the 100-500 mg/L range. High suspended-sediment concentrations tended to occur in streams in areas dominated by range and agricultural land due to the high erodibility of soils in these areas (fig 43B).
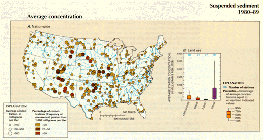 A.,B.
A.,B.
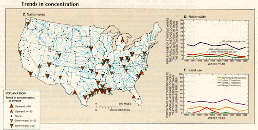 C.,D.,E.
C.,D.,E.
Downward trends (37 stations) in suspended-sediment concentration, which greatly outnumbered upward trends (5 stations), occurred mostly at stations in the south-central part of the country and along the Gulf Coast (fig. 43C). Nationally, the percentage of stations having annual average concentrations greater than 100 mg/L declined from 37 to 31 percent (fig. 43D). The steepest declines in the percentage of stations having annual average concentrations greater than 500 mg/L occurred in areas dominated by range and agricultural land (fig. 43E), and it is likely that increased efforts in soil conservation during 1980-89 contributed at least in part to these trends. The U.S. Soil Conservation Service (1989) estimates that sheet and rill erosion on rural land, a category that includes crop and range land cover, decreased by 13 percent between 1982 and 1987.