National Water-Quality Assessment (NAWQA) Project
Go to:
BACK TO CIRCULAR 1291 Materials Page
U.S. Geological Survey Circular 1291
Appendix 8A. Analytical Approach and Methods for Pesticides in Stream Water
This appendix provides information on the approach and methods used for analysis of stream-water data provided in USGS Circular 1291, Pesticides in the Nation's Streams and Ground Water, 1992 – 2001: The Quality of Our Nation's Waters (Gilliom and others, 2006).
Study design and site selection: Stream water was sampled as part of the NAWQA Occurrence and Distribution Survey. The goal of the survey is to “characterize, in a nationally consistent manner, the broad-scale geographic and seasonal distributions of water-quality conditions in relation to major contaminant sources and background conditions” (Gilliom and others, 1995, p. 1).
Stream-water sites in each Study Unit were selected for sampling in accordance with the NAWQA national design strategy (Gilliom and others, 1995). Two general types of stream-water sites were selected for monitoring—“Integrator Sites” and “Indicator Sites.” Typically, three to five Integrator Sites and four to eight Indicator Sites were selected in each Study Unit. Integrator Sites represent water quality in large basins that is often affected by mixed land uses or a wide variety of natural influences. Indicator Sites represent water quality in smaller, more homogeneous basins that is often affected by a single land use or a more limited number of natural influences. Integrator sites are located at key points (such as the outlet) in the drainage network of a Study Unit. Indicator Sites are located in targeted areas of relatively homogeneous land use and physiographic conditions (Gilliom and others, 1995, p. 11-12). Targeted land uses include agricultural, urban, and, to a much lesser degree, undeveloped forest or rangeland (U.S. Geological Survey, 1999, p. 30). The stream-water sites sampled for the NAWQA program were not selected to be a statistically representative sample of the Nation’s land use or any other factor. Rather, the NAWQA national design is intended to provide a framework for comparing water-quality among sites to improve the understanding of the relation between water quality and land use, pesticide use, soils, climate, and other natural or human influences.
Not all NAWQA stream-water sites were sampled for pesticides. Most water samples collected for the analysis of pesticides (hereafter termed “pesticide samples”) were collected at a subset of sites termed “Intensive Fixed Sites.” Sampling for pesticides at these sites was more frequent during periods when pesticides were applied or pesticide occurrence in streams was expected (Gilliom and others, 1995, p. 17). Typically, one to two Integrator Sites and one to four Indicator Sites were intensively sampled for pesticides in each Study Unit. In some Study Units, other types of NAWQA sites occasionally had a sufficient number of pesticide samples to estimate an annual distribution of concentrations and these sites were included in the national assessment.
Classification of sites by land use: A nationally consistent approach was used to classify stream sites by land use. Each site was classified into one of four land-use classes—agricultural, urban, undeveloped, and mixed—depending on the amount of agricultural, urban, and undeveloped land within the watershed. This approach does not take into account all factors that affect water quality at the site, but instead aims to characterize the land use associated with the streamflow (water) at the site. An initial determination of land use within each watershed was made using an enhanced version of the 1992 National Land Cover Data (NLCD), which classifies land use for each 30-meter-square area of land in the conterminous U.S. The original and enhanced versions of the NLCD are described, respectively, by Vogelmann and others (2001) and Nakagaki and Wolock (2005). The percentage of the watershed corresponding to each of three aggregated land-use categories was calculated: total agricultural land (including row crops, pasture, and orchards), total urban land (including residential and commercial-industrial), and total undeveloped land (including forest and rangeland). The criteria specified in table A (also shown as table 3-1 of Gilliom and others (2006) were applied to classify each site on the basis of the percentage of land in these three aggregated categories.
Table A. National criteria for classifying NAWQA stream sites according to the dominant land use(s) in their watersheds.
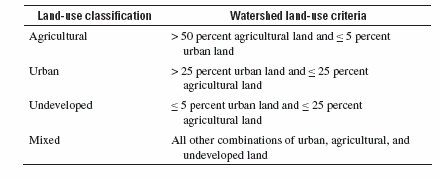
A few sites (fewer than 5 percent) had their classifications adjusted if one or more land uses makes a disproportionately large or small contribution (relative to its percentage of land area in the watershed) to streamflow at the site because of water management practices or natural variations in precipitation and runoff. This is especially common in the arid west, where water resources are heavily managed.
Sample collection and processing: Pesticide samples generally were collected for one year at each site using a combination of fixed-interval and extreme-flow sampling (Gilliom and others, 1995, p. 16). Fixed-interval sampling (also called fixed-frequency sampling) is the collection of water samples at regular intervals of time and results in a time series of samples where the number of days between samples is approximately the same. For the fixed-interval sampling, two to four samples generally were collected each month during seasonal periods of high pesticide use and runoff and one to two samples were collected each month during other periods. The intensive seasonal sampling period typically ranged from three to nine months (Gilliom and others, 1995, p. 17). Additional samples were collected during periods of extreme high or low streamflow (usually high flows). Extreme-flow sampling is intended to supplement fixed-interval sampling by targeting hydrologic conditions that are important, but occur infrequently and are unlikely to be sampled solely on the basis of fixed-interval sampling. Samples were collected more frequently at some sites where short-term fluctuations were a concern. Most sites were intensively sampled for only 1 year, whereas others had multiple years of data collection for pesticides.
Streamflow-weighted, depth- and width-integrated water samples were collected using standard U.S. Geological Survey (USGS) methods (Shelton, 1994, p. 14-17). All sample collection and processing equipment that came in contact with sample water was constructed of Teflon, glass, aluminum, or stainless steel. Equipment was cleaned with a dilute solution of phosphate-free detergent and rinsed with deionized water and pesticide-grade methanol. Water samples were filtered using pre-combusted glass-fiber filters with a nominal 0.7-µm pore diameter to remove suspended particulate matter and collected in baked amber glass bottles. Filtered samples were placed on ice in coolers and shipped to the USGS National Water Quality Laboratory (NWQL) in Denver, Colorado for analysis. Additional details on collection and processing methods are described by Shelton (1994).
The quality of the stream-water pesticide data collected for the NAWQA program was monitored using quality-control procedures presented in Mueller and others (1997). The field quality-control program included the collection of field blank water samples to asses potential contamination, replicate water samples to asses variability, and field matrix spikes to assess potential pesticide degradation or matrix effects. Contamination in field blank water samples is summarized in Martin and others (1999). Variability in replicate water samples is summarized in Martin (2002). Pesticide recovery in laboratory reagent spikes and field matrix spikes is summarized in Martin (1999).
Pesticides, analytical methods, and reporting levels: Most NAWQA water samples were analyzed for 75 pesticides and 8 pesticide degradates (Gilliom and others, 2006, Appendix 1A). These pesticides account for approximately 78 percent of the total amount (by weight) of pesticides used for agriculture in the United States in 1997 (Gilliom and others, 2006, figure 3-4) and a substantial portion of urban and suburban use. Water samples were analyzed for pesticides at the USGS NWQL. Two analytical methods were extensively used during the first cycle (1992-2001) of the NAWQA program—GCMS and HPLC. Both methods use solid-phase extraction to remove pesticides from filtered water samples.
The GCMS method was the primary analytical method used by all Study Units. The GCMS method provides low-level analyses of 47 pesticides or degradates by gas chromatography/mass spectrometry and selected-ion monitoring (Zaugg and others, 1995). The pesticide acetochlor was added to the GCMS method in June 1994 (Lindley and others, 1996); consequently some sites used in the national assessment do not have analyses of acetochlor. USGS personnel refer to the GCMS method as NWQL schedules 2001 or 2010.
The HPLC method was used extensively by Study Units that began investigations in 1991 and 1994. The HPLC method provides low-level analyses of 36 additional pesticides or degradates by high-performance liquid chromatography and photodiode-array detection (Werner and others, 1996). Approximately one third of the sites used in the national assessment did not have analyses by HPLC. USGS personnel refer to the HPLC method as NWQL schedules 2050 or 2051.
Low-level detections of pesticides by these analytical methods are not censored at the reporting level. All detections (pesticides conclusively identified by retention time and spectral characteristics) are quantified and concentrations less than the reporting level are reported with an “E” remark to indicate that the concentration--but not the presence--is estimated. In addition, concentrations less than the lowest calibration standard, concentrations extrapolated above the highest calibration standard, or samples diluted to bring concentrations within the range of the calibration standards also are remarked “E” (Oblinger Childress and others, 1999, p. 8-10). Concentrations in excess of 20.0 µg/L determined by GCMS or in excess of 1.50 µg/L determined by HPLC generally are above the calibration curve of the method and are remarked “E.” Data users should infer that the uncertainty in the concentration for a concentration remarked “E” is expected to be relatively larger than that for a concentration without an “E” remark.
Any detections of five pesticides analyzed by GCMS (azinphos-methyl, carbaryl, carbofuran, deethylatrazine, and terbacil) and six pesticides analyzed by HPLC (aldicarb, aldicarb sulfone, aldicarb sulfoxide, chlorothalonil, dichlobenil, and DNOC) are reported with an “E” remark, regardless of concentration. These pesticides have lower or more variable recovery relative to the other pesticides analyzed by the method (Zaugg and others, 1995, p. 35; Werner and others, 1996, pp. 27, 34; U.S. Geological Survey, 1998). The presence or absence of an “E” remark for any pesticide compound was not considered in the statistical analysis of the concentration data.
The types and numerical values of reporting levels have changed through time (table B). A reporting level is the “less than” concentration used to indicate a pesticide nondetection (for example: < 0.005 µg/L). Procedures used by NWQL to set reporting levels, the types of reporting levels, and considerations for data analysis are discussed in Oblinger Childress and others (1999). All reporting levels for pesticide nondetections reported by the NWQL (except those for samples with analytical difficulties resulting in “raised reporting levels,” discussed below) were changed to the maximum value of the long-term method detection level (LT-MDL) for water years 1992-2002 (MAXLTMDL in table B). The maximum value of the LT-MDL was determined by review of NWQL records of annual values of LT-MDL.
Table B. Reporting levels for pesticides analyzed in NAWQA water samples, 1992 –2002 water years.
[Pesticide and degradates are sorted by analytical method and NWIS parameter code. GCMS, gas chromatography/mass spectrometry; HPLC, high-performance liquid chromatography; NWIS parameter code, the number used to identify a pesticide in the U.S. Geological Survey National Water Information System; CRL, common reporting level for the indicated water year, MAXCRL, maximum common reporting level for water years 1992–2002; MAXLTMDL, maximum long-term method detection level for water years 1992–2002; µg/L, micrograms per liter; na, pesticide not analyzed]
| Method | NWIS parameter code | Pesticide | CRL 1992 (µg/L) | CRL 1993 (µg/L) | CRL 1994 (µg/L) | CRL 1995 (µg/L) | CRL 1996 (µg/L) | CRL 1997 (µg/L) | CRL 1998 (µg/L) | CRL 1999 (µg/L) | CRL 2000 (µg/L) | CRL 2001 (µg/L) | CRL 2002 (µg/L) | MAXCRL (µg/L) | MAXLTMDL (ug/L) |
| GCMS | 04024 | Propachlor | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.01 | 0.01 | 0.01 | 0.005 |
| GCMS | 04028 | Butylate | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 |
| GCMS | 04035 | Simazine | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.011 | 0.005 | 0.011 | 0.006 |
| GCMS | 04037 | Prometon | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.015 | 0.015 | 0.018 | 0.007 |
| GCMS | 04040 | Deethylatrazine | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.006 | 0.006 | 0.006 | 0.003 |
| GCMS | 04041 | Cyanazine | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.018 | 0.018 | 0.018 | 0.009 |
| GCMS | 04095 | Fonofos | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.0027 | 0.0027 | 0.003 | 0.001 |
| GCMS | 34253 | alpha-HCH | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.0046 | 0.0046 | 0.0046 | 0.002 |
| GCMS | 34653 | p,p'-DDE | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.0025 | 0.0025 | 0.006 | 0.001 |
| GCMS | 38933 | Chlorpyrifos | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.005 | 0.005 | 0.005 | 0.003 |
| GCMS | 39341 | gamma-HCH | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.002 |
| GCMS | 39381 | Dieldrin | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.0048 | 0.0048 | 0.0048 | 0.002 |
| GCMS | 39415 | Metolachlor | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.013 | 0.013 | 0.013 | 0.006 |
| GCMS | 39532 | Malathion | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.027 | 0.027 | 0.027 | 0.014 |
| GCMS | 39542 | Parathion | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.007 | 0.01 | 0.01 | 0.005 |
| GCMS | 39572 | Diazinon | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.005 | 0.005 | 0.005 | 0.003 |
| GCMS | 39632 | Atrazine | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.007 | 0.007 | 0.007 | 0.004 |
| GCMS | 46342 | Alachlor | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.0024 | 0.0045 | 0.0045 | 0.002 |
| GCMS | 49260 | Acetochlor | na | na | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.0041 | 0.006 | 0.006 | 0.003 |
| GCMS | 82630 | Metribuzin | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.006 | 0.006 | 0.006 | 0.003 |
| GCMS | 82660 | 2,6-Diethylaniline | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.0017 | 0.006 | 0.006 | 0.003 |
| GCMS | 82661 | Trifluralin | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.009 | 0.009 | 0.009 | 0.005 |
| GCMS | 82663 | Ethalfluralin | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.009 | 0.009 | 0.009 | 0.005 |
| GCMS | 82664 | Phorate | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.011 | 0.011 | 0.011 | 0.006 |
| GCMS | 82665 | Terbacil | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.034 | 0.034 | 0.034 | 0.017 |
| GCMS | 82666 | Linuron | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.035 | 0.035 | 0.035 | 0.018 |
| GCMS | 82667 | Parathion-methyl | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.003 |
| GCMS | 82668 | EPTC | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 |
| GCMS | 82669 | Pebulate | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.0016 | 0.0041 | 0.0041 | 0.002 |
| GCMS | 82670 | Tebuthiuron | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.016 | 0.016 | 0.016 | 0.008 |
| GCMS | 82671 | Molinate | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.0016 | 0.0016 | 0.004 | 0.001 |
| GCMS | 82672 | Ethoprop | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.005 | 0.005 | 0.005 | 0.002 |
| GCMS | 82673 | Benfluralin | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.01 | 0.01 | 0.01 | 0.005 |
| GCMS | 82674 | Carbofuran | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.02 | 0.02 | 0.02 | 0.01 |
| GCMS | 82675 | Terbufos | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.017 | 0.017 | 0.017 | 0.009 |
| GCMS | 82676 | Pronamide | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.0041 | 0.0041 | 0.0041 | 0.002 |
| GCMS | 82677 | Disulfoton | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.021 | 0.021 | 0.021 | 0.011 |
| GCMS | 82678 | Triallate | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.0023 | 0.0023 | 0.0023 | 0.001 |
| GCMS | 82679 | Propanil | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.011 | 0.011 | 0.011 | 0.005 |
| GCMS | 82680 | Carbaryl | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.041 | 0.041 | 0.041 | 0.021 |
| GCMS | 82681 | Thiobencarb | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.0048 | 0.0048 | 0.0048 | 0.002 |
| GCMS | 82682 | Dacthal | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.002 |
| GCMS | 82683 | Pendimethalin | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.01 | 0.022 | 0.022 | 0.011 |
| GCMS | 82684 | Napropamide | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.007 | 0.007 | 0.007 | 0.003 |
| GCMS | 82685 | Propargite | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.023 | 0.023 | 0.023 | 0.011 |
| GCMS | 82686 | Azinphos-methyl | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.05 | 0.05 | 0.05 | 0.02 |
| GCMS | 82687 | cis-Permethrin | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.006 | 0.006 | 0.006 | 0.003 |
| HPLC | 04029 | Bromacil | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.06 | 0.09 | 0.09 | 0.09 | 0.04 |
| HPLC | 38442 | Dicamba | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.043 | 0.043 | 0.11 | 0.11 | 0.05 |
| HPLC | 38482 | MCPA | na | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.17 | 0.17 | 0.17 | 0.08 | 0.2 | 0.2 | 0.1 |
| HPLC | 38487 | MCPB | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.14 | 0.14 | 0.13 | 0.13 | 0.26 | 0.26 | 0.13 |
| HPLC | 38501 | Methiocarb | na | 0.026 | 0.026 | 0.026 | 0.026 | 0.026 | 0.026 | 0.026 | 0.026 | 0.07 | 0.07 | 0.07 | 0.03 |
| HPLC | 38538 | Propoxur | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.08 | 0.12 | 0.12 | 0.12 | 0.06 |
| HPLC | 38711 | Bentazon | na | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.035 | 0.035 | 0.05 | 0.05 | 0.03 |
| HPLC | 38746 | 2,4-DB | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.24 | 0.24 | 0.1 | 0.1 | 0.25 | 0.25 | 0.13 |
| HPLC | 38811 | Fluometuron | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.06 | 0.06 | 0.06 | 0.06 | 0.03 |
| HPLC | 38866 | Oxamyl | na | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.16 | 0.16 | 0.08 |
| HPLC | 39732 | 2,4-D | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.15 | 0.15 | 0.11 | 0.11 | 0.16 | 0.16 | 0.08 |
| HPLC | 39742 | 2,4,5-T | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.04 | 0.04 | 0.07 | 0.07 | 0.04 |
| HPLC | 39762 | 2,4,5-TP | na | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.06 | 0.025 | 0.025 | 0.06 | 0.03 |
| HPLC | 49235 | Triclopyr | na | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.25 | 0.25 | 0.25 | 0.07 | 0.07 | 0.25 | 0.04 |
| HPLC | 49236 | Propham | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.09 | 0.22 | 0.22 | 0.11 |
| HPLC | 49291 | Picloram | na | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.09 | 0.09 | 0.09 | 0.04 |
| HPLC | 49292 | Oryzalin | na | 0.019 | 0.019 | 0.019 | 0.019 | 0.019 | 0.31 | 0.31 | 0.31 | 0.28 | 0.28 | 0.31 | 0.14 |
| HPLC | 49293 | Norflurazon | na | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.042 | 0.042 | 0.042 | 0.042 | 0.021 |
| HPLC | 49294 | Neburon | na | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.07 | 0.017 | 0.07 | 0.07 | 0.03 |
| HPLC | 49296 | Methomyl | na | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.47 | 0.47 | 0.24 |
| HPLC | 49297 | Fenuron | na | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.07 | 0.07 | 0.07 | 0.07 | 0.03 |
| HPLC | 49299 | DNOC | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.42 | 0.42 | 0.42 | 0.25 | 0.25 | 0.42 | 0.13 |
| HPLC | 49300 | Diuron | na | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.06 | 0.056 | 0.12 | 0.12 | 0.06 |
| HPLC | 49301 | Dinoseb | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.06 | 0.09 | 0.09 | 0.09 | 0.04 |
| HPLC | 49302 | Dichlorprop | na | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.05 | 0.12 | 0.12 | 0.06 |
| HPLC | 49303 | Dichlobenil | na | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 1.2 | 1.2 | 0.07 | 0.049 | 0.09 | 1.2 | 0.05 |
| HPLC | 49304 | Dacthal monoacid | na | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.039 | 0.07 | 0.07 | 0.07 | 0.04 |
| HPLC | 49305 | Clopyralid | na | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.23 | 0.23 | 0.23 | 0.42 | 0.42 | 0.42 | 0.21 |
| HPLC | 49306 | Chlorothalonil | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.48 | 0.48 | 0.48 | 0.13 | 0.25 | 0.48 | 0.07 |
| HPLC | 49308 | 3-Hydroxycarbofuran | na | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.11 | 0.11 | 0.11 | 0.11 | 0.05 |
| HPLC | 49311 | Bromoxynil | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.04 | 0.07 | 0.07 | 0.07 | 0.03 |
| HPLC | 49312 | Aldicarb | na | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.55 | 0.55 | 0.21 | 0.21 | 0.21 | 0.55 | 0.1 |
| HPLC | 49313 | Aldicarb sulfone | na | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 |
| HPLC | 49314 | Aldicarb sulfoxide | na | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.021 | 0.27 | 0.27 | 0.14 |
| HPLC | 49315 | Acifluorfen | na | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.035 | 0.09 | 0.05 | 0.05 | 0.09 | 0.04 |
| HPLC | 61188 | Chloramben methyl ester | na | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.42 | 0.42 | 0.14 | 0.14 | 0.21 | 0.42 | 0.11 |
Water-quality data: The analytical data summarized in USGS Circular 1291 (Gilliom and others, 2006) were compiled March 29, 2002. Pesticide analytical data were available through January 15, 2002. Data were subjected to extensive automated data-checking routines and were further checked to ensure the exclusion of quality-control samples. Verification of unusually high pesticide concentrations was sought from Study-Unit personnel.
Some analytical values were not used in the national assessment. The maximum “common” reporting level for water years 1992–2002 (MAXCRL in table B) was used to identify nondetections of pesticides that could be attributed to sample analytical difficulties such as matrix interference. An analytical value reported as a nondetection at a concentration greater than the maximum common reporting level (a “raised reporting level”) was deleted from the data set. The common reporting level is the most frequently occurring reporting level for each pesticide and water year (for example, CRL 1995 in table B) in the water-quality data set. Analytical data remarked “V” also were deleted from the data set. Analytical data remarked “V” by Study-Unit personnel indicates that a value was affected by a significant amount of contamination (U.S. Geological Survey, 1997).
Selection of sites and samples for the national assessment: A 1-year period of pesticide data was selected for each site to describe an annual distribution of pesticide concentrations and to avoid biasing results to sites with multiple years of data collection. The initial population of sites for the assessment consisted of all Intensive Fixed Sites (see section “Study design and site selection”) and other NAWQA stream-water sites with 12 or more pesticide samples. Samples were sorted by date and time for each site and the number of days between samples and the number of pesticides measured at each site were determined. The times series of samples at each site was visually examined and sites with fewer than 8 samples per 1-year period or with large gaps between samples during the pesticide-use season were deleted. In general, the selected 1-year period was the year with the most samples and analytes (all pesticides at a site had the same selected period). The selected 1-year period began on the first day of a month but usually did not coincide with the start of a water year or calendar year. The population of stream-water sites selected for use in the national assessment was composed of 153 Intensive Fixed Sites and 33 other NAWQA stream-water sites. The number of pesticide samples collected at each site during the selected 1-year periods ranged from 8 to 50 and the median was 22.5. The mean number of days between samples at each site during the selected 1-year periods ranged from 6.6 to 36.5 and the median was 15.3.
Summary statistics: Time-weighted detection frequencies, percentiles of concentration, and mean concentrations were calculated from the 1-year time series of concentrations available for each site. Calculations were done by site and by land-use class. PROC UNIVARIATE of the Statistical Analysis System (version 6 or version 8) was used to calculate all summary statistics by site and by land-use class (SAS Institute Inc., 2002).
Calculation of annual time-weighted detection frequency, percentiles of concentration, and mean concentration by stream-water site: Detection frequencies, percentiles of concentration, and mean concentrations for each site were calculated by weighting each concentration in the 1-year time series by the amount of time it was used to represent the pesticide concentration in the stream. Specifically, the weights were computed as the amount of time extending from one-half the time interval between the sample and the previous sample (or to the beginning of the 1-year period) plus one-half the time interval between the sample and the subsequent sample (or to the end of the 1-year period) divided by the total time in the 1-year period. Sample weights sum to 1.
Time-weighted estimates account for the more frequent sample collection during the pesticide-use season and yield an estimate of the percentage of time (percentage of the 1-year period) concentrations occurred. Simple sample-based estimates (as opposed to time-weighted estimates) fail to account for the more frequent sample collection during the pesticide-use season and, consequently, may provide annual estimates of pesticide occurrence biased to concentrations that occur during the pesticide-use season.
Time-weighted frequencies of detection for each pesticide at each site were calculated for four detection thresholds: (1) detections greater than or equal to 1 µg/L, (2) detections greater than or equal to 0.1 µg/L, (3) detections greater than or equal to 0.01 µg/L, and (4) detections at any concentration—some as low as 0.001 micrograms per liter. The frequency of detection is the sum of the sample weights of samples in which the specific pesticide compound was detected at or above the threshold. The summed sample weights were multiplied by 100% to express frequency of detection as a percentage.
Some of the concentration thresholds used for frequency of detection were less than the maximum value of the long-term method detection level used to indicate pesticide nondetection (MAXLTMDL in table B). In these cases, the detection frequencies may underestimate the true percentage of samples with concentrations greater than the threshold and detection frequencies are preceded with a ">" symbol. The maximum value of the long-term method detection level was greater than the 0.01 µg/L threshold for all pesticides analyzed by HPLC, therefore, detection frequency at this threshold was not calculated for pesticides analyzed by HPLC.
Time-weighted percentiles (95th, 90th, 75th, and 50th) were calculated for each pesticide at each site. Computation of time-weighted percentiles of concentration can be conceptualized as ranking the concentration data from low to high and cumulatively summing the sample weights. The pth percentile corresponds to the concentration where the cumulative summed weight is p/100. For example, the 95th percentile concentration corresponds to the concentration where the cumulative summed weight equals 0.95. Time-weighted percentiles provide an estimate of the percentage of time a particular concentration occurred in stream water. For example, a time-weighted 95th-percentile atrazine concentration of 0.1 µg/L indicates that concentrations of atrazine were less than or equal to 0.1 µg/L for 95 percent of the year (approximately 347 days). Percentiles also may be interpreted as the percentage of the year where concentrations were greater than a given concentration. For example, a time-weighted 95th-percentile atrazine concentration of 0.1 µg/L indicates that concentrations of atrazine were greater than or equal to 0.1 µg/L for 5 percent of the year (approximately 18 days).
Time-weighted percentiles of concentration were computed using one of five approaches dependent on the amount of nondetected values and the results for percentiles calculated using the fourth approach. The approaches were used in the following order of preference:
Approach 1. If there were no nondetections, percentiles were calculated as described in the preceding paragraph.
Approach 2. If more than 0 percent but less than 67 percent of the weighted data were nondetections and the data set contained at least 20 values and at least 10 nondetected values, then the probability-plotting / regression method (MR) described by Helsel and Cohn (1988) was used.
Approach 3. If the criteria for approach 2 were not met but 10 percent or less of the weighted data were nondetections, nondetected values were replaced with one-half of the MAXLTMDL (U.S. Environmental Protection Agency, 1998, p. 4.7-2)
Approach 4. If the criteria for approach 3 were not met, percentiles were calculated without regard to nondetections (as described in approach 1) and any calculated percentile less than the MAXLTMDL (table B) was considered a nondetection.
Approach 5. A fifth approach was used for selected percentiles if the percentile calculated by the fourth approach was a nondetection. For the fifth approach, all nondetections were ranked lower than any detection at the site and the selected percentile was recalculated as described in approach 1. Any recalculated percentile less than the lowest detection at the site was considered a nondetection. The fifth approach facilitated the calculation of percentiles for some pesticides (such as carbaryl) with numerous detections less than the MAXLTMDL.
Note that percentiles calculated using approaches 1, 2, 3, and 5 may result in concentrations less than the MAXLTMDL (table B).
Time-weighted mean concentrations were computed for each site and pesticide using one of four approaches dependent on the amount of nondetected values. The approaches were used in the following order of preference:
Approach 1. If there were no nondetections, mean concentration was calculated as the sum of the sample weights times the sample concentrations.
Approach 2. If 10 percent or less of the weighted data were nondetections, nondetected values were replaced with one-half of the MAXLTMDL (U.S. Environmental Protection Agency, 1998, p. 4.7-2) and the mean concentration was calculated as the sum of the sample weights times the sample concentrations.
Approach 3. If more than 10 percent but less than 67 percent of the weighted data were nondetections and the data set contained at least 20 values and at least 10 detected values, then the probability-plotting / regression method (MR) described by Helsel and Cohn (1988) was used.
Approach 4. If the criteria in approach 3 were not met, mean concentration was calculated without regard to nondetections (as described in approach 1) and the calculated mean was remarked “<” to indicate that the time-weighted mean is less than the calculated value.
Note that approaches 2 and 3 are reversed for percentiles and means. If less than 10 percent of the weighted data are nondetections, the substitution method is preferable to the probability plotting method for the mean but not for percentiles. Also note that mean concentrations less than the MAXLTMDL (table B) may be calculated if concentrations less than the MAXLTMDL were measured at the site (see section “Pesticides, analytical methods, and reporting levels”).
A “maximum” concentration was determined for each site. The concentrations are the maximum measured concentrations in the selected 1-year period of data. Concentrations greater than the value reported as maximum may have been measured at the stream-water site but are not reported because they were not measured during the 1-year period selected to describe the annual distribution of concentrations. In addition, the probability is low that a water sample was collected at the time of the annual maximum concentration for any pesticide or site. Consequently, the maximum concentrations should be interpreted as a lower bound for the true maximum concentrations.
Calculation of annual time-weighted detection frequency and percentiles of concentration by land-use class: Detection frequencies and percentiles for land-use classes were computed using the same data and similar procedures as for individual sites. Sample weights were calculated by site as described previously and pooled by land-use class. Sample weights in the pooled data sets were normalized by the number of sites in the pooled data set (each site in the land-use class had an equal influence on the class statistic). Subsequent procedures for calculation of time-weighted detection frequencies were identical to those used for individual sites. Time-weighted percentiles of concentration for land-use classes were computed by ranking all nondetections lower than any detection in the land-use group and cumulatively summing the sample weights. Any calculated percentile less than the lowest detection in the land-use group was considered a nondetection.
Additional methods and approach: Additional information on methods and approach for stream-water analyses for individual figures in Circular 1291 are provided in the online supplemental information section of each figure.
Gilliom, R.J., Alley, W.M., and Gurtz, M.E., 1995, Design of the National Water-Quality Assessment Program: Occurrence and distribution of water-quality conditions: U.S. Geological Survey Circular 1112, 33 p. Available at: http://water.usgs.gov/pubs/circ/circ1112/
Gilliom, R.J., Barbash, J.E., Crawford, C.G., Hamilton, P.A., Martin, J.D., Nakagaki, Naomi, Nowell, L.H., Scott, J.C., Stackelberg, P.E., Thelin, G.P., and Wolock, D.M., 2006, Pesticides in the Nation's streams and ground water, 1992-2001--The quality of our Nation's waters: U.S. Geological Survey Circular 1291, 171p. Available at: URL http://water.usgs.gov/nawqa/pnsp/pubs/circ1291/
Helsel, D.R., and Cohn, T.A., 1988, Estimation of descriptive statistics for multiply censored water quality data: Water Resources Research, v. 24, no. 12, p. 1997-2004.
Lindley, C.E., Stewart, J.T., and Sandstrom, M.W., 1996, Determination of low concentrations of acetochlor in water by automated solid-phase extraction and gas chromatography with mass-selective detection: Journal of the AOAC International, v. 79, no. 4, p. 962-966.
Martin, J.D., 1999, Quality of pesticide data for environmental water samples collected for the National Water-Quality Assessment Program, 1992-96, and examples of the use of quality-control information in water-quality assessments: accessed March 21, 2006 at http://water.usgs.gov/nawqa/pnsp/pubs/qcsummary.html
Martin, J.D., 2002, Variability of pesticide detections and concentrations in field replicate water samples collected for the National Water-Quality Assessment Program, 1992-97: U.S. Geological Survey Water-Resources Investigations Report 01-4178, 84 p. Available at: http://in.water.usgs.gov/newreports/martin-pest.pdf
Martin, J.D., Gilliom, R.J., and Schertz, T.J., 1999, Summary and evaluation of pesticides in field blanks collected for the National Water-Quality Assessment Program: 1992-95: U.S. Geological Survey Open-File Report 98-412, 102 p. Available at: http://water.usgs.gov/nawqa/pnsp/pubs/files/ofr98412.pdf
Mueller, D.K., Martin, J.D., and Lopes, T.J., 1997, Quality-control design for surface-water sampling in the National Water-Quality Assessment Program: U.S. Geological Survey Open-File Report 97-223, 17 p. Available at: http://water.usgs.gov/nawqa/protocols/OFR97-223/index.html
Nakagaki, Naomi, and Wolock, D.M., 2005, Estimation of agricultural pesticide use in drainage basins using land cover maps and county pesticide data: U.S. Geological Survey Open-File Report 2005-1188, 46 p.
Oblinger Childress, C.J., Foreman, W.T., Connor, B.F., and Maloney, T.J., 1999, New reporting procedures based on long-term method detection levels and some considerations for interpretations of water-quality data provided by the U.S. Geological Survey National Water Quality Laboratory: U.S. Geological Survey Open-File Report 99-193, 19 p. Available at: http://water.usgs.gov/owq/OFR_99-193/index.html
SAS Institute Inc., 2002, Base SAS: SAS Institute Inc., accessed January 13, 2002 at http://www.sas.com/products/base/index.html
Shelton , L.R., 1994, Field guide for collecting and processing stream-water samples for the National Water-Quality Assessment Program: U.S. Geological Survey Open-File Report 94-455, 42 p. Available at: http://water.usgs.gov/nawqa/pnsp/pest.rep/sw-t.html
U.S. Environmental Protection Agency, 1998, Guidance for data quality assessment--Practical methods for data analysis: U.S. Environmental Protection Agency, Office of Research and Development, EPA/600/R-96/084, variable pagination. Available at: http://www.epa.gov/region10/www/offices/oea/epaqag9.pdf
U.S. Geological Survey, 1997, NWIS--New remark code (V) for water-quality data: Office of Water Quality Technical Memorandum 97.08. Available at: http://water.usgs.gov/admin/memo/QW/qw97.08.html
_____, 1998, Changes in reporting levels and data qualifiers for selected pesticides and degradation products in schedules 2050 and 2051: National Water Quality Laboratory Technical Memorandum 98.03A. Available at: http://nwql.usgs.gov/Public/tech_memos/nwql.98-03A.html
_____, 1999, The quality of our Nation’s waters--Nutrients and pesticides: U.S. Geological Survey Circular 1225, 82 p. Available at: http://water.usgs.gov/pubs/circ/circ1225/
Vogelmann, J.E., Howard, S.M., Yang, L., Larson, C.R., Wylie, B.K., and VanDriel, Nick, 2001, Completion of the 1990’s national land cover dataset for the conterminous United States from Landsat Thematic Mapper data and ancillary data sources: Photogrammetric Engineering and Remote Sensing, v. 67, p. 650–662.
Werner, S.L., Burkhardt, M.R., and DeRusseau, S.N., 1996, Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory--Determination of pesticides in water by Carbopak-B solid-phase extraction and high-performance liquid chromatography: U.S. Geological Survey Open-File Report 96-216, 42 p. Available at: http://wwwnwql.cr.usgs.gov/Public/pubs/OFR96-216/OFR96-216.html
Zaugg, S.D., Sandstrom, M.W., Smith, S.G., and Fehlberg, K.M., 1995, Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory--Determination of pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring: U.S. Geological Survey Open-File Report 95-181, 49 p. Available at: http://wwwnwql.cr.usgs.gov/Public/pubs/OFR95-181/OFR95-181.html
Pesticide names and analytical methods are presented in Appendix 1A.
Information on sampling sites and their characteristics is presented in Appendix 5A.
Downloadable concentration data are presented in Appendix 6A.
Summary statistics by land-use class and by stream-water site are presented in Appendix 7A.
For more information, contact:
Jeffrey D. Martin
U.S. Geological Survey
NAWQA Pesticide Synthesis Project
5957 Lakeside Boulevard
Indianapolis , IN 46278-1996
voice: (317) 290-3333 x148
fax: (317) 290-3313
email: jdmartin@usgs.gov