National Water-Quality Assessment (NAWQA) Project
Go to:
Appendix 2 - Properties affecting the transport and fate of selected pesticide compounds.
[Pesticide compounds selected are those detected most frequently in NAWQA samples (see figs. 4-2 and 4-4), as well as several that were detected infrequently, despite extensive use. All values measured at (or estimated for) 25°C, except for those shown in italics. Unless noted otherwise, (1) values for octanol-water partition coefficient (Kow, dimensionless), soil organic carbon-water partition coefficient (Koc), water solubility (Sw) and Henry's law constant (KH) are from Mackay and others (1997); (2) transformation half-lives in soil and water were measured in the laboratory (rather than in the field) at neutral pH in the dark, and obtained from the U.S. Department of Agriculture (2005); and (3) all are recommended values selected by the compilation authors when more than one value was available from the literature. Compounds are listed in the same order as in figures 4-2 and 4-4. Numbers of significant figures are identical to those given in original sources. kJ/mol, kilojoule per mole; mg/L, milligram per liter; mL/g, milliliter per gram; NA, data not available from any of the references consulted; Pa•m3/mol, pascal-cubic meter per mole; >, greater than.]
Compound |
log Kow |
log Koc (Koc in mL/g) |
Sw (mg/L) |
log KH (KH in Pa•m3/mol) |
Half-life for transformation |
||||||
In aerobic soil |
In water |
||||||||||
Agricultural herbicides and degradates detected most frequently in water |
|||||||||||
Atrazine |
2.75 |
2.00 |
30 |
-3.54 |
146 |
1742 |
|||||
Deethylatrazine (DEA) |
21.3 |
31.90 |
42700 |
5-4.12 |
6170 |
NA |
|||||
Metolachlor |
3.13 |
2.26 |
430 |
-2.63 |
26 |
7410 |
|||||
Cyanazine |
2.22 |
2.3 |
171 |
-6.52 |
817 |
9>200 |
|||||
Alachlor |
2.8 |
2.23 |
240 |
-2.7 |
1020.4 |
11640 |
|||||
Acetochlor |
123.0 |
132.38 |
12,14223 |
12,15-2.15 |
13,1514 |
162300 |
|||||
Metribuzin |
14,171.60 |
1.72 |
14,171,000 |
17,18-5.31 |
172 |
9>200 |
|||||
Bentazon |
192.80 |
201.54 |
14,17500 |
21-3.7 |
2235 |
23>200 |
|||||
EPTC |
3.2 |
2.3 |
370 |
0.00988 |
247 |
9>200 |
|||||
Trifluralin |
5.34 |
4.14 |
250.5 |
1.00 |
169 |
26>32 |
|||||
Molinate |
3.21 |
1.92 |
970 |
-0.839 |
2721 |
9>200 |
|||||
Norflurazon |
172.45 |
172.55 |
1734 |
17-4.46 |
130 |
9>200 |
|||||
Urban herbicides detected most frequently in water |
|||||||||||
Simazine |
2.18 |
2.11 |
5 |
-3.46 |
891 |
28>32 |
|||||
Prometon |
2.99 |
2.54 |
750 |
-4.05 |
932 |
9>200 |
|||||
Tebuthiuron |
81.79 |
172.1 |
292400 |
17-4.88 |
1050 |
30>2700 |
|||||
2,4-D |
2.81 |
171.68 |
890 |
-3.61 |
312.3 |
7732 |
|||||
Diuron |
2.78 |
2.6 |
40 |
-3.17 |
372 |
>500 |
|||||
Dacthal (DCPA) |
324.28 |
173.75 |
170.5 |
17-0.66 |
3316 |
9>200 |
|||||
Bromacil |
2.11 |
1.86 |
815 |
-4.89 |
275 |
34>30 |
|||||
Insecticides detected most frequently in water |
|||||||||||
Diazinon |
3.3 |
2.76 |
60 |
-1.39 |
39 |
140 |
|||||
Chlorpyrifos |
4.92 |
3.78 |
0.73 |
0.0374 |
30.5 |
29 |
|||||
Carbofuran |
2.32 |
2.02 |
351 |
-4.30 |
11 |
35289 |
|||||
Carbaryl |
2.36 |
2.36 |
120 |
-4.35 |
17 |
11 |
|||||
Malathion |
2.8 |
3.26 |
145 |
-2.64 |
<1 |
366.3 |
|||||
Dieldrin37 |
5.20 |
4.08 |
0.17 |
0.0492 |
NA |
3830 |
|||||
Organochlorine pesticide compounds detected most frequently in bed sediment and fish tissue |
|||||||||||
p,p'-DDE |
5.7 |
5.0 |
0.04 |
0.900 |
NA |
38>44,000 |
|||||
p,p'-DDD |
5.5 |
5.0 |
0.05 |
-0.194 |
NA |
3910,000 |
|||||
p,p'-DDT |
6.19 |
5.4 |
0.0055 |
0.37 |
NA |
405000 |
|||||
o,p'-DDE |
5.8 |
85.58 |
0.1 |
0.405 |
NA |
NA |
|||||
o,p'-DDD |
6.0 |
85.36 |
250.10 |
41-2.7 |
NA |
39NA |
|||||
o,p'-DDT |
42NA |
42NA |
0.026 |
-0.460 |
NA |
NA |
|||||
cis-Chlordane |
6.0 |
5.5 |
0.056 |
-0.466 |
NA |
43>7.2x107 |
|||||
trans-Chlordane |
6.0 |
5.5 |
0.056 |
-0.582 |
NA |
44>10,000 |
|||||
Nonachlor |
455.66 |
454.86 |
450.06 |
45-1.69 |
NA |
NA |
|||||
Oxychlordane |
462.6 |
462.48 |
46200 |
46-1.52 |
NA |
NA |
|||||
Dieldrin37 |
5.20 |
4.08 |
0.17 |
0.0492 |
NA |
3830 |
|||||
Heptachlor epoxide |
5.0 |
4.0 |
0.35 |
460.51 |
NA |
NA |
|||||
Pentachloroanisole |
465.66 |
464.62 |
460.2 |
462.91 |
NA |
NA |
|||||
Hexachlorobenzene |
325.31 |
174.7 |
320.0062 |
471.69 |
NA |
48>26,000 |
|||||
Chlorothalonil |
2.64 |
3.2 |
0.6 |
1.77 |
NA |
9>200 |
|||||
Dicamba |
2.21 |
171.11 |
4500 |
-3.66 |
4928 |
9>200 |
|||||
Parathion-methyl (Methyl parathion) |
3.0 |
3.7 |
25 |
-1.68 |
503.3 |
41 |
|||||
Pendimethalin |
175.2 |
174.13 |
170.275 |
170.0899 |
1300 |
9>200 |
|||||
Terbufos |
4.48 |
2.70 |
5 |
0.39 |
5 |
1.9 |
|||||
1Measured by Khan (1978) in a solution containing 0.5 g/L fulvic acid.
2Computed from Koc value using Kow-vs.-Koc regression for "triazines" from Sabljić and others (1995).
3Koc value measured on a clay loam soil (1.3% organic carbon) by Seybold and Mersie (1996), the study—among those having measured Koc for both DEA and atrazine—reporting the Koc value for atrazine (140 mL/g) that was closest to the value selected for atrazine (100 mL/g) by Mackay and others (1997).
4Value from Ciba-Geigy, Ltd., cited by Bayless (2001)
5Estimated by (1) computing estimates of KH for both DEA and atrazine (KH,est[DEA] and KH,est[atr], respectively) using the bond-contribution method of Meylan and Howard (1991); and (2) scaling from the value measured for atrazine (KH,meas[atr]) using the following equation:
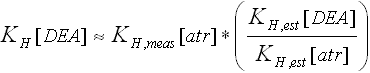
6Measured by Kruger and others (1997) in the aerobic soil (sandy clay loam, 0.6% organic carbon) for which the half-life for atrazine reported by the authors and adjusted to 25°C (191 d) was most similar to the value selected by the U.S. Department of Agriculture (2005) for atrazine at 25°C (146 d). Half-lives for both atrazine and DEA in soil were adjusted to 25°C from 24°C using an activation energy of 48.4 kJ/mol, measured by Baer and Calvet (1999) for atrazine disappearance in an aerobic silt loam (1.1% organic carbon).
7Rate extrapolated to 25°C using data reported by Cavalier and others (1991) for disappearance of the pesticide in ground-water samples (pH = 7.4-7.6) at 15 and 22°C, based on the experiment with the highest initial pesticide concentration (and thus maximum analytical sensitivity).
8Sole value reported by the U.S. Department of Agriculture (2005) database authors, but not "selected."
9Classified as "stable" with respect to hydrolysis (U.S. Department of Agriculture, 2005), meaning that the compound exhibits "less than 10% reaction after 30 days at 25°C" (Cheryl Sutton, U.S. Environmental Protection Agency Office of Pesticide Programs, Environmental Fate and Effects Division, personal communication, 1/4/05).
10Half-life interpolated to 25°C from rates of alachlor disappearance from a sandy loam soil (0.7% organic carbon) at 10, 20 and 30°C reported by Zimdahl and Clark (1982).
11Rate extrapolated to 25°C from data reported by Cavalier and others (1991) for alachlor disappearance in ground water (pH = 7.4-7.6) at 15 and 22°C, based on the experiment with the highest initial alachlor concentration (and thus maximum analytical sensitivity). Value consistent with half-life estimate of ">100 d" at 20°C in deionized water reported by Gan and others (2002).
12Source: U.S. Environmental Protection Agency (1994).
13Median value among those reported by U.S. Environmental Protection Agency (1994).
14Measured at 20°C.
15Temperature of measurement not given.
16Source: Zheng and Ye (2001), cited by Carlson (2003). Consistent with half-life of ">100 d" at 20°C in deionized water reported by Gan and others (2002).
17Source: U.S. Department of Agriculture (2005).
18Adjusted from value measured at 20°C to 25°C using equation provided by Mackay and others (2000), and an enthalpy of vaporization (ΔHv) of 46.8 kJ/mol, based on KH data from 197 compounds (Staudinger and Roberts, 2001).
19Source: Sabljić and others (1995). Form of molecule (salt vs. benzothiadiazine) not given.
20Measured using the sodium salt of bentazon, rather than the benzothiadiazine form (U.S. Department of Agriculture, 2005).
21Computed from dimensionless value given by Tiktak (1999). Temperature of measurement not given, but similarity of value to that computed from vapor pressure and solubility (both obtained from EXTOXNET) suggests that it may have been 20°C.
22Median value among 19 measurements of bentazon disappearance rates in a variety of soils (pH= 6.1-7.7) over a temperature range of 20-23°C (Huber and Otto, 1994; Thorstensen and Lode, 2001), after adjusting all values to 25°C using an activation energy of 90 kJ/mol for bentazon disappearance, measured for a sandy soil with 4.90% organic carbon (Tiktak, 1999).
23Statement by Huber and Otto (1994) that "hydrolytically, bentazon is nearly stable...at pH...7" presumed to be equivalent to U.S. Environmental Protection Agency's definition of "stable" (see footnote 9), although temperature of measurement not given.
24"[H]alf-life in moist loam soil at 21 to 27°C is approximately one week" (Mackay and others, 1997).
25According to Mackay and others (1997), "the reported values for this quantity vary considerably [0.05 - 40 mg/L for trifluralin; 0.10 - 0.24 mg/L for o,p'-DDD]; whereas this selected value represents the best judgement of the authors...it may be subject to large error."
26Computed from rate constant (maximum value) provided by U.S. Department of Agriculture (2005).
27According to Mackay and others (1997), "half-life is approximately 3 weeks in moist loam soils at 21-27°C."
28For biodegradation in pond water. Source: Tucker and Boyd (1981), cited by Mackay and others (1997).
29Arithmetic mean of the two values reported by U.S. Department of Agriculture (2005), neither of which was "selected."
30Estimated from assumption that "no hydrolysis in 64 days" at 51°C and pH 3, 6 and 9 (U.S. Department of Agriculture, 2005) implies less than 5% reaction. Extrapolated to 25°C using the minimum activation energy (Ea) for the transformation in soil of methabenzthiazuron (35.6 kJ/mol), the urea herbicide most similar in structure to tebuthiuron among those for which Ea values appear to be available (FOCUS, 1997). (No Ea values appear to be available for the disappearance of either methabenzthiazuron or tebuthiuron in water.)
31Average of values computed for two soils, based on data reported by Veeh and others (1996).
32Source: Syracuse Research Corporation CHEMFATE Database.
33Source: Wettasinghe and Tinsley (1993).
34Transformation rate given for pH 5 and 9 only (U.S. Department of Agriculture, 2005).
35Transformation rate measured at pH 6.2 (U.S. Department of Agriculture, 2005).
36Value measured at pH 7, rather than the pH (5) at which the value recommended by the U.S. Department of Agriculture (2005) was measured.
37Dieldrin is both an insecticide and a product of aldrin transformation (Nowell and others, 1999), and was one of the pesticide compounds detected most frequently in bed sediments, as well as in water.
38Reaction rate measured in 5% acetonitrile solution, extrapolated to 27°C from higher temperatures, and interpolated to pH 5 by Wolfe and others (1977).
39No distinction made between o,p' and p,p' isomers for this parameter by the U.S. Department of Agriculture (2005), but reported half-life was identical to that provided by Ellington and others (1987a) for p,p'-DDD, suggesting that the U.S. Department of Agriculture (2005) value was for the p,p' isomer only.
40Reaction rate measured in 5% acetonitrile solution, extrapolated to 27°C from higher temperatures, and interpolated to pH 7 by Wolfe and others (1977).
41Source: Suntio and others (1988).
42Available estimation methods appear inadequate for distinguishing between ortho and para isomers.
43Source: Ellington and others (1987a).
44Estimated from assumption that "zero degradation...during five days" at 85°C and pH 7 (Ellington and others, 1987b) implies less than 10% reaction (Ellington and others, 1988). Extrapolated to 25°C using the activation energy for the transformation of γ-HCH in borate buffer at pH 9 (84.6 kJ/mol), reported by Ngabe and others (1993).
45Source: Nowell and others (1999). No distinction made between cis and trans isomers.
46Source: Nowell and others (1999).
47Computed for 25°C using regression equation from Staudinger and Roberts (2001).
48Estimated from assumption that "zero hydrolysis observed after 13 days at 85°C and pH 7 (Ellington and others, 1987b) implies less than 10% reaction (Ellington and others, 1988). Extrapolated to 25°C using the activation energy for the transformation of γ-HCH in borate buffer at pH 9 (84.6 kJ/mol), reported by Ngabe and others (1993).
49Interpolated to 25°C from data for a clay soil (1.5% organic matter) by Comfort and others (1992).
50Extrapolated to 25°C from value measured at 30°C in a silt loam soil (organic carbon content not given) by Lichtenstein and Schulz (1964) after 12 days of reaction (the longest reaction period examined by the authors), using the activation energy (Ea) measured by Lartiges and Garrigues (1995) for methyl parathion disappearance in unfiltered river water at pH 7.3 (26 kJ/mol). (No Ea values appear to be available for methyl parathion disappearance from aerobic soil.)
REFERENCES CITED
Baer, U. and Calvet, R., 1999, Fate of soil applied herbicides: Experimental data and prediction of dissipation kinetics. J.Environ.Qual. 28:1765-1777.
Bayless. E.R., 2001, Atrazine retention and degradation in the vadose zone at a till plain site in central Indiana. Ground Water 39(2): 169-180.
Carlson, D.L., 2003, Environmental transformations of chloroacetamide herbicides: Hydrolysis and reaction with iron pyrite. Ph.D. dissertation, Johns Hopkins University, Baltimore, MD.
Cavalier, T. C., Lavy, T. L. and Mattice, J. D., 1991, Persistence of selected pesticides in ground-water samples. Ground Water, 29(2): 225-231.
Comfort, S.D., Inskeep, W.P. and Macut, R.E., 1992, Degradation and transport of dicamba in a clay soil. J.Env.Qual. 21: 653-658.
Ellington, J. J., Stancil, F. E., Jr., Payne, W. D. and Trusty, C., 1987a, Measurement of hydrolysis rate constants for evaluation of hazardous waste land disposal, Volume II: Project Summary. USEPA, EPA/600/3-87/019. Athens, Georgia. 6 p.
Ellington, J. J., Stancil, F. E., Jr., Payne, W. D. and Trusty, C., 1987b, Measurement of hydrolysis rate constants for evaluation of hazardous waste land disposal: Volume II. Data on 54 chemicals. USEPA, EPA/600/3-87/019. Athens, Georgia. 152 p.
Ellington, J. J., Stancil, F. E., Jr., Payne, W. D. and Trusty, C., 1988, Interim protocol for measuring hydrolysis rate constants in aqueous solutions: Project Summary. USEPA, EPA/600/S3-88/014. Athens, Georgia. 4 p.
FOCUS (Forum for the co-ordination of pesticide fate models and their use) Soil Modelling Work group, 1997, Soil persistence models and EU registration. Publication number 7617/V1/96.
Gan, J., Qang, Q., Yates, S.R., Koskinen, W.C. and Jury, W.A., 2002, Dechlorination of chloroacetanilide herbicides by thiosulfate salts. Proc.Nat.Acad.Sci. 99(8): 5189-5194.
Huber, R. and Otto, S., 1994, Environmental behavior of bentazon herbicide. Rev.Environ.Contam.Toxicol. 137: 111-134.
Khan, S. U., 1978, Kinetics of hydrolysis of atrazine in aqueous fulvic acid solution. Pesticide Science, 9: 39-43.
Kruger, E.L., Rice, P.J., Anhalt, J.C., Anderson, T.A. and Coats, J.R., 1997, Comparative fates of atrazine and deethylatrazine in sterile and nonsterile soils. J.Env.Qual. 26: 95-101.
Lartiges, S.B. and Garrigues, P.P., 1995, Degradation kinetics of organophosphorus and organonitrogen pesticides in different waters under various environmental conditions. Env.Sci.Technol. 29(5): 1246-1254.
Lichtenstein, E.P. and Schulz, K.R., 1964, The effects of moisture and microorganisms on the persistence and metabolism of some organophosphorous insecticides in soils, with special emphasis on parathion. J.Econ.Entomol. 57: 618-627.
Mackay, D., Shiu, W-Y., Ma, K-C., 1997, Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, Volume V. Pesticide Chemicals. New York, NY: Lewis Publishers. 812 p.
Mackay, D.M., Shiu, W.Y. and Ma, K.C., 2000, Henry's law constant In: Handbook of Property Estimation Methods for Chemicals - Environmental and Health Sciences, Boethling, R.S. and Mackay, D., eds. New York, NY: Lewis Publishers, pp. 69-87.
Meylan, W.M. and Howard, P.H., 1991, Bond contribution method for estimating Henry's law constants. Env.Toxicol.Chem. 10: 1283-1293.
Ngabe, B., Bidleman, T. F. and Falconer, R. L., 1993, Base hydrolysis of alpha- and gamma-hexachlorocyclohexanes. Environmental Science and Technology, 27(9): 1930-1933.
Nowell, L.H., Capel, P.D. and Dileanis, P.D., 1999, Pesticides in Stream Sediment and Aquatic Biota - Distribution, Trends and Governing Factors. Boca Raton, FL: CRC Press, 1001 p.
Sabljić, A., Gusten, H., Verhaar, H. and Hermens, J., 1995, QSAR modeling of soil sorption. Improvements and systematics of log Koc vs. log Kow correlations. Chemosphere 31(11/12): 4489-4514.
Seybold, C.A. and Mersie, W., 1996, Adsorption and desorption of atrazine, deethylatrazine, deisopropylatrazine, hydroxyatrazine, and metolachlor in two soils from Virginia. J.Env.Qual. 25: 1179-1185.
Staudinger, J. and Roberts, P.V., 2001, A critical compilation of Henry's law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 44: 561-576.
Suntio, L. R., Shiu, W. Y., Mackay, D., Seiber, J. N. and Glotfelty, D., 1988, Critical review of Henry's Law constants for pesticides. Reviews of Environmental Contamination and Toxicology, 103: 1-59.
Thorstensen, C.W. and Lode, O., 2001, Laboratory degradation studies of bentazone, dichlorprop, MCPA, and propiconazole in Norwegian soils. J.Env.Qual. 30: 947-953.
Tiktak, A., 1999, Modeling non-point source pollutants in soils - Applications to the leaching and accumulation of pesticides and cadmium. Ph.D. dissertation, University of Amsterdam, Department of Physical Geography and Soil Science, 224 p.
Tucker, C.S. and Boyd, C.E., 1981, Relationships between pond sediments and simazine loss from waters of laboratory systems. J.Aquat. Plant Manag. 19: 55.
U.S. Department of Agriculture, 2005, Pesticide Properties Database. U.S. Department of Agriculture-Agricultural Research Service.
U.S. Environmental Protection Agency, 1994, Pesticide Environmental Fate Database One-Line Summary-Acetochlor. Office of Pesticide Programs, Environmental Fate and Effects Division, Washington, D.C. (Last updated 9/9/94.)
Veeh, R.H., Inskeep, W.P. and Camper, A.K., 1996, Soil depth and temperature effects on microbial degradation of 2,4-D. J.Environ.Qual. 25: 5-12.
Wettasinghe A and Tinsley I.J., 1993, Degradation of dacthal and its metabolites in soil. Bull. Environ. Cont. Tox. 50: 226-231.
Wolfe, N.L., Zepp, R.G., Paris, D.F., Baughman, G.L. and Hollis, R.C., 1977, Methoxychlor and DDT degradation in water: Rates and products. Env.Sci.Technol., 11[12]: 1077-1081.
Zheng, H. and Ye, C., 2001, Huanjing Huaxue 20: 168-171. (Cited by Carlson, 2003.)
Zimdahl, R.L. and Clark, S.K., 1982, Degradation of three acetanilide herbicides in soil. Weed Sci. 30: 545-548.