National Water-Quality Assessment (NAWQA) Project
Go to:
FIELD GUIDE FOR COLLECTING AND PROCESSING
STREAM-WATER SAMPLES FOR THE NATIONAL
WATER-QUALITY ASSESSMENT PROGRAM
By Larry R. Shelton
U.S. GEOLOGICAL SURVEY
Open-File Report 94-455
Sacramento, California
1994
U.S. DEPARTMENT OF THE INTERIOR
BRUCE BABBITT, Secretary
U.S. GEOLOGICAL SURVEY
GORDON P. EATON, Director
Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government.
For printed copies of the published report contact:
CONTENTS
Conversion Factors
______________________________________________________________________
Multiply By To obtain
----------------------------------------------------------------------
foot (ft) 0.3048 meter
foot per second (ft/s) 0.3048 meter per second
gallon (gal) 3.785 liter
inch (in.) 25.4 millimeter
inch per second (in/s) 25.4 millimeter per second
pound, avoirdupois (lb) 4.536 kilogram
pound per square inch (lb/in2) 6.895 kilopascal
square mile (mi2) 2.590 square kilometer
______________________________________________________________________
Temperature is given in degrees Celsius ( C), which can be converted to
degrees Fahrenheit ( F) by the following equation:
F=1.8( C)+32
Abbreviations
cm, centimeter g/L, gram per liter L, liter uL, microliter um, micrometer uS/cm, microsiemens per centimeter at 25 degrees Celsius mg/L, milligram per liter mg/ L, milligram per microliter mL, milliliter mL/min, milliliter per minute mm, millimeter DIW deionized water DOC dissolved organic carbon EDI equal-discharge increment EWI equal-width increment FA filtered acidified FC filtered chilled FU filtered untreated GCMS gas chromatography/mass spectrometer HPLC high-pressure liquid chromatography N normal OCALA Quality Water Service Unit, USGS, Ocala, Florida PPB parts per billion PVC polyvinyl chloride RC raw (unfiltered) chilled RU raw (unfiltered) untreated SOC suspended-organic carbon SPE solid-phase extraction TOC toxic organic compounds VOC volatile organic compound
Acronyms
ASTM American Society for Testing and Materials HIF Hydrologic Instrumentation Facility, USGS, Mississippi NAWQA National Water-Quality Assessment NWQL National Water Quality Laboratory OSW Office of Surface Water OWQ Office of Water Quality TWRI Techniques of Water-Resources Investigations USGS U.S. Geological Survey WRD Water Resources Division YSI Yellow Springs Instrument
GLOSSARY
Basic Fixed Sites -- Sites on streams at which streamflow is measured and samples are collected for temperature, salinity, suspended sediment, major ions and metals, nutrients, and organic carbon to assess the broad-scale spatial and temporal character and transport of inorganic constituents of stream water in relation to hydrologic conditions and environmental settings.
Bed-Sediment and Tissue Studies -- Assessment of concentrations and distributions of trace elements and hydrophobic organic contaminants in stream bed sediment and tissues of aquatic organisms to identify potential sources and assess spatial distribution.
Depth-integrating sampler -- A sampler that will integrate and represent the area of a stream section.
Discharge-weighted samplers -- A sampler that will isokinetically represent the stream flow.
Ecological Studies -- Studies of biological communities habitat characteristics to evaluate the effects of physical and chemical characteristics of water and hydrologic conditions on aquatic biota and to determine how biological and habitat characteristics differ among environmental settings in Study Units.
Equal-width increment (EWI) sampling -- A composite sample across a section of stream with equal spacing between verticals and equal transit rates within each vertical that yields a representative sample of stream conditions.
Gaging station -- A fixed site on a stream or river where hydrologic and environmental data are collected.
Indicator Sites -- Stream sampling sites located at outlets of drainage basins with relatively homogeneous land use and physiographic conditions. Basins are as large and representative as possible, but still encompassing primarily one Environmental Setting (typically, 50 to 500 km2).
Integrator Site -- Stream sampling sites located downstream of drainage basins that are large and complex and often contain multiple Environmental Settings. Most Integrator Sites are on major streams with drainage basins that include a substantial portion of the Study Unit area (typically, 10 to 100 percent).
Intensive Fixed Sites -- Basic Fixed Sites with increased sampling frequency during selected seasonal periods and analysis of dissolved pesticides for 1 year. Most Study Units have one or two integrator Intensive Fixed Sites and one to four indicator Intensive Fixed Sites.
Isokinetic sampling -- The water entering the sampler is hydrodynamically equivalent (velocity, area, and direction) to the portion of the stream being sampled.
Occurrence and Distribution Assessment -- Assessment of the broad-scale geographic and seasonal distributions of water-quality conditions for surface and ground water of a Study Unit in relation to major contaminant sources and background conditions.
Solid-phase extraction (SPE) -- A procedure to isolate specific organic compounds onto a bonded silica extraction column.
Study Unit -- A major hydrologic system of the United States in which NAWQA studies are focused. NAWQA Study Units are geographically defined by a combination of ground- and surface-water features and usually encompass more than 10,000 km2 of land area. The NAWQA design is based on assessment of 60 Study Units, which collectively cover a large part of the Nation, encompass the majority of population and water use, and include diverse hydrologic systems that differ widely in natural and human factors that affect water quality.
Water-Column Studies -- Assessment of physical and chemical characteristics of stream water, including suspended sediment, dissolved solids, major ions and metals, nutrients, organic carbon, and dissolved pesticides, in relation to hydrologic conditions, sources, and transport.
By Larry R. Shelton
Abstract The U.S. Geological Survey's National Water-Quality Assessment program includes extensive data-collection efforts to assess the quality of the Nation's streams. These studies require analyses of stream samples for major ions, nutrients, sediments, and organic contaminants. For the information to be comparable among studies in different parts of the Nation, consistent procedures specifically designed to produce uncontaminated samples for trace analysis in the laboratory are critical. This field guide describes the standard procedures for collecting and processing samples for major ions, nutrients, organic contaminants, sediment, and field analyses of conductivity, pH, alkalinity, and dissolved oxygen. Samples are collected and processed using modified and newly designed equipment made of Teflon to avoid contamination, including nonmetallic samplers (D-77 and DH-81) and a Teflon sample splitter. Field solid-phase extraction procedures developed to process samples for organic constituent analyses produce an extracted sample with stabilized compounds for more accurate results. Improvements to standard operational procedures include the use of processing chambers and capsule filtering systems. A modified collecting and processing procedure for organic carbon is designed to avoid contamination from equipment cleaned with methanol. Quality assurance is maintained by strict collecting and processing procedures, replicate sampling, equipment blank samples, and a rigid cleaning procedure using detergent, hydrochloric acid, and methanol.
INTRODUCTION The National Water-Quality Assessment (NAWQA) program of the U.S. Geological Survey (USGS) is designed to assess the status and trends in the quality of the Nation's ground- and surface-water resources and to develop an understanding of the major factors that affect water-quality conditions (Hirsch and others, 1988; Leahy and others, 1990; Gilliom and others, 1994). The design is based on balancing the unique assessment requirements of individual hydrologic systems with a nationally consistent design structure that incorporates a multiscale, interdisciplinary approach. Investigations of water quality in 60 major hydrologic basins and aquifer systems, referred to as NAWQA Study Units, form the building blocks of the program.
The Occurrence and Distribution Assessment, described in Gilliom and others (1994), is the largest and most important component of the first intensive study phase in each Study Unit. The goal of the Occurrence and Distribution Assessment is to characterize, in a nationally consistent manner, the broad- scale geographic and seasonal distribution of water-quality conditions in relation to major contaminant sources and background conditions. The national study design for streams has three interrelated components. Water-Column Studies assess the occurrence and distribution of major ions, nutrients, and dissolved pesticides and their relation to hydrologic conditions, sources, and transport. Bed-Sediment and Tissue Studies assess the occurrence and spatial distribution of trace elements and hydrophobic organic contaminants. Ecological Studies evaluate the physical, chemical, and biological characteristics of streams relative to environmental settings. Sampling designs for these components coordinate sampling of varying intensity and scope at common sites. The glossary at the front of this report includes brief definitions of the NAWQA study components, indicated throughout the report with capital first letters, and other key terms.
This report describes standard methods for collecting and processing water-column samples from streams as part of the Occurrence and Distribution Assessment component of the NAWQA program. Complimentary methods and procedures are described for collecting and processing biological tissues (Crawford and Luoma, 1992; Meador and others, 1993), bed sediments (Shelton and Capel, 1994), inorganic constituents (Horowitz and others, 1994), ground water (Koterba and others 1995), and volatile organic compounds (Shelton, 1996). The methods and techniques described in this report are intended to enable investigators to meet the specific goals of the NAWQA program and are oriented to specific USGS equipment, practices, and support facilities. However, they also can be adapted for use by other Federal and state agencies, as well as by other programs of the USGS.
The procedures described conform to methods presented in the USGS Techniques of Water-Resources Investigations (TWRI) series and in the technical memorandums of the Office of Water Quality (OWQ) and the Office of Surface Water (OSW) of the USGS (see appendix A). The procedures are based, in part, on guidelines released by the OWQ and on a field manual prepared by M.A. Sylvester and others of the U.S. Geological Survey (see appendix B). New material has been added for selected procedures, and some guidelines have been modified to conform with the NAWQA Study-Unit design guidelines. The development of new and improved field techniques is a continuing process; therefore, this field guide will require periodic updating. If these updates outline a different or improved procedure, investigators in each Study Unit will evaluate the effect on the resulting data. Compatibility with previously collected data is essential for the duration of each project.
Trade names used in connection with equipment or supplies do not constitute an endorsement of the product. References are made throughout this document to the U.S. Geological Suvey's National Water Quality Laboratory (NWQL); Quality Water Service Unit at Ocala, Florida (OCALA); and the Hydrologic Instrumentation Facility (HIF).
OVERVIEW OF WATER-COLUMN STUDY DESIGN Water-Column Studies in NAWQA focus on assessing physical and chemical characteristics of stream water, including suspended sediment, dissolved solids, major ions and metals, nutrients, organic carbon, and dissolved pesticides, and on relating these characteristics to hydrologic conditions, sources, and transport. The sampling designs for Water-Column Studies rely on coordinated sampling of varying intensity and scope at two general types of sites, Integrator Sites and Indicator Sites. Integrator Sites are chosen to represent water-quality conditions of streams and rivers in heterogeneous large basins that often are affected by complex combinations of land-use settings, point sources, and natural influences. Indicator Sites, in contrast, are chosen to represent water-quality conditions of streams in relatively homogeneous and usually smaller basins associated with specific individual environmental settings (for example, a particular combination of land-use and geological setting).
Water-column conditions are assessed by three primary sampling strategies employed at the selected Integrator and Indicator Sites:
Intensive Fixed-Site assessments characterize seasonal and short-term temporal variability of general water quality and constituent transport and determine the occurrence and seasonal patterns in concentrations and transport of dissolved pesticides; and
Synoptic studies are investigations of the geographic distribution of selected water-quality characteristics in greater detail during specific seasons and in relation to sources.
Each Study Unit typically has three to five integrator Basic Fixed Sites and four to eight indicator Basic Fixed Sites. Intensive Fixed Sites usually are composed of one or two Integrator Sites and one to four Indicator Sites. Samples are collected from each site at fixed intervals and at extreme flows. The analytical strategy for samples collected at Basic Fixed Sites is summarized in table 1; the strategy for samples collected at Intensive Fixed Sites is the same, but with the addition of laboratory analyses of dissolved pesticides (table 2).
Table 1. Analytical strategy for Basic Fixed Sites
_________________________________________________________
Field measurements
_________________________________________________________
Dissolved oxygen
pH and Alkalinity
Specific conductance (consider hourly)
Temperature (hourly for 1 year)
_________________________________________________________
Laboratory analyses
_________________________________________________________
Suspended sediment
Major constituents:
Dissolved solids
Major ions and metals:
Calcium
Chloride
Fluoride
Iron
Magnesium
Manganese
Potassium
Silica
Sodium
Sulfate
Nutrients
Nitrogen:
Total
Total dissolved
Ammonia
Nitrite
Nitrate
Phosphorus:
Total
Total dissolved
Ortho
Organic carbon:
Suspended
Dissolved
_________________________________________________________
Analytical strategy for Intensive Fixed Sites in
addition to the Basic Fixed Site analyses
_________________________________________________________________________
Field measurements
_________________________________________________________________________
Specific conductance (hourly or daily for 1 year)
_________________________________________________________________________
Laboratory analyses: dissolved pesticides
_________________________________________________________________________
Amides:
Alachlor Napropamide Propachlor
Metolachlor Pronamide Propanil
Carbamates:
Aldicarb Carbofuran, 3-Hydroxy Pebulate
Aldicarb sulfone EPTC Propham
Aldicarb sulfoxide Methiocarb Propoxure
Butylate Methomyl Thiobencarb
Carbaryl Molinate Triallate
Carbofuran Oxamyl
Chloropheoxy herbicides:
2,4-D (acid) MCPA 2,4,5-T
Dichlorprop (2,4-DP) MCPB Triclopyr
2,4-DB Silvex (2,4,5-TP)
Dinitroanalins:
Benfluralin Oryzalin Trifluralin
Ethafluralin Pendimethalin
Organochlorines:
Chlorothalonil p,p'DDE alpha-HCH
Dacthal (DCPA) Dichlobenil gamma-HCH
Decthal (mono acid) Dieldrin
Organophosphates:
Azinphos-methyl Disulfoton Methyl parathion
Chlorpyrifos Ethoprop Parathion
Diazinon Fonofos Phorate
Dimethoate Malathion Terbufos
Pyrethroids:
cis-Permethrin
Triazine herbicides:
Atrazine Cyanazine Prometon
Atrazine, desethyl Metribuzin Simazine
Uracils:
Bromacil Terbacil
Ureas:
Fenuron Fluometuron Neburon
Diuron Linuron Tebuthiuron
Miscellaneous:
Actifluorfen Dicamba 1-Napthol
Bentazon 2,6-Diethylanaline Norflurazon
Bromoxynil Dinoseb Picloram
Chloramben DNOC Propargite
Clopyralid Esfenvalerate
________________________________________________________________________
PREPARATION FOR SAMPLE COLLECTION
SITE SELECTION
All Basic Fixed Sites and Intensive Fixed Sites should be at or near streamflow gaging stations because stream discharges associated with chemical-constituent concentrations are needed to compute constituent transport and to evaluate relations between streamflow and water-quality characteristics (Gilliom and others, 1994). The sample collection site should not be more than a few hundred feet from the site of the gage, unless no appreciable inflow is between the sampling site and the gaging station.Criteria for selecting a site for water-sample collection are different from those for selecting a site for measurement of streamflow. Greater accuracy in computing constituent transport may be attained by selecting a cross section based on sediment-transport and mixing characteristics rather than hydraulic measurements such as velocity. Collection sites should be located in relatively straight channel reaches where the flow is uniform. Collecting samples directly in a ripple or from ponded or sluggish water should be avoided. Sites upstream or downstream of confluences or point sources also should be avoided to minimize problems caused by backwater effects or poorly mixed flows. Samples collected directly downstream from a bridge can be contaminated from the bridge structure or runoff from the road surface.
SAMPLING EQUIPMENT The standard samplers used in the NAWQA program for collecting water samples include the DH-81, D-77 TM, D-77 Bag, and weighted- and open-bottle samplers with Teflon or glass components. These samplers will collect representative water-chemistry samples in most stream environments; however, their limitations must be carefully considered when collecting isokinetic samples (see OSW technical memorandum 94.05, appendix A). For a more thorough discussion of the proper use of each sampler, see the "Collection Methods" section.
Knowledgeable, independent field judgement is essential for collecting a sample representative of the stream chemistry. The following information should be considered before making a decision on which sampler to use:
Table 3. List of equipment and supplies for sampling and processing stream-water samples
[NWQL, National Water Quality Laboratory; DIW, deionized water; DO, dissolved oxygen; SPE, solid-phase extraction; ft, foot; gal, gallon; g/L, gram per liter; in. inch; L, liter; mL, milliliter; mm, millimeter; um, micrometer; uS/cm, microsiemens per centimeter at 25 degrees Celsius]
SAMPLING
Splitting
The following list of suppliers produce the above equipment to the recommended specifications. Listing a specific company is not intended as an endorsement.
* QW Service Unit ** Army COE WES *** Hydro Inst (HIF)
4500 SW 40th Ave 3909 Halls Ferry Rd Stennis Space Ctr
Ocala, FL 34474 Vicksburg, MS 39180 MI 39529-6000
904 237-5514 601 634-2721 800 382-0634
# Cole-Parmer Inst. ## Geotech
7425 N Oak Park Ave 1441 W. 46th Ave
Chicago, IL 60648 Denver, CO 80211
800 323-4340 303 433-7101
@ Wildlife Supply Co @@ Gilson Co Inc
301 Cass St. P.O. Box 677
Saginaw. MI 48602 Worthing, OH 43085
517 799-8100 800 444-1508
HAND-HELD SAMPLERS The DH-81 or an open-bottle sampler should be used when streamflow conditions permit the stream to be waded. The DH-81 sampler consists of a polypropylene collar screwed onto a plastic-coated wading rod. The collar is notched to accept the D-77-type Teflon cap and nozzle assembly, which is locked in the sampling position. A 1- or 3-L Teflon bottle can be used with the appropriate cap-bottle adaptor. The DH-81 can use a 1/4- or 5/16-in. Teflon nozzle. An open-bottle sampler is the simplest means of collecting a water sample. An uncapped glass or Teflon bottle is submerged in the stream by hand (see "Weighted Bottle" section).
SUSPENDED SAMPLERS The following suspended samplers can collect depth-integrated samples when used within their recommended limits. These samplers are suspended from, and lowered into, the stream by a rope or cable and usually require the use of additional equipment because of their weight (see "Support Equipment" section).
D-77 TM This 75-lb sampler (epoxy coated to prevent trace-element contamination) collects large-volume (nearly 3 L) samples. This sampler is approved for flow velocities from 2.0 to 8.0 ft/s, though some instability has been noted in turbulent flow velocities exceeding 6.0 ft/s. Depth limitations of the D-77 TM sampler are dependent on a combination of depth and velocity, preventing the sampler from overfilling when used with the recommended transit rate and the required nozzles. The recommended operating depth is between 3 and 15 ft. The Teflon cap has standard Mason-jar threads to make it compatible with a large glass sample bottle. The older Teflon caps require a Teflon cap-bottle adaptor when using the 3-L bottle. Caps purchased after July 1994 will not require the use of the cap-bottle adaptor. The recommended nozzle sizes for the D-77 TM sampler is 1/4- and 5/16-in. and must be made of Teflon. However, in extremely high flows or when sampling depths cause the 3-L sample bottle to overfill in a single vertical, a 3/16-in. nozzle can be used. This sampler was counterweighted during manufacturing for specific-sample bottle use. Always check the balance to ensure that the sampler is level when fitted with an empty bottle, cap, adapter, and nozzle.
D-77 Bag This sampler is designed to collect large-volume (up to 8 L) samples. Counterweights suspended below this sampler allow for sample collection in streams where depths exceed the limits of the D-77 TM and where the combination of depth and velocity cause other samplers to overfill. The sampler uses the standard D-77 Teflon cap and nozzles. Teflon bags attached to the cap are held in place by a large rigid bottle, a frame, or both. The bag enables this sampler to collect larger volume samples. Prototypes currently are being tested. It is not known if the sampler collects samples isokinetically; the pressure inside the nozzle must be greater then the pressure outside the nozzle for the bag to fill. This sampler is difficult to use because of the collapsible bag and the sediment is hard to remove. Its use is not recommended when velocities are less than 2 ft/s and should be used only when the D-77 TM is inappropriate. Plans and operating instructions for this sampler are available from HIF. Document the use of this sampler.
Under Ice Under-ice samplers that use the D-77 bottle, cap, and nozzle are currently being developed. The sampler is based on the DH-81 design and pivots into a vertical position, allowing it to go through an 8-in. ice hole. Contact the HIF for more information. Until this sampler is available, a DH-81 or a weighted bottle sampler should be used. A hand-held ice chisel should be used when making holes in the ice to avoid leaving residual contamination from the power equipment.
Weighted bottle A weighted bottle is a simple way to collect a water sample in slow moving streams. Weights are added to an uncapped glass or Teflon bottle suspended from a rope for depth sampling. The sampler can be handmade (plans are available from HIF) and consists of a plastic basket or frame with a weight attached that holds a specific-size bottle. These samplers do not collect a depth-integrated, isokinetic sample; the sampling depth is mainly dependent on the capacity and inside diameter of the bottle opening. However, a representative sample usually can be collected from shallow streams when the suspended sediment is distributed uniformly in the vertical and the velocity is less than 2.0 ft/s. These samplers are most appropriate where differences in water-quality distribution within the cross section of the stream are insignificant. A Teflon Kemmerer sampler can be used to composite depth-integrated samples from various depths. A Kemmerer sampler is a 4- by 18-in. tube with end caps that close by means of a messenger and entrap a 4.2-L water sample inside. This sampler collects a point sample from a specific depth. Composite several point samples from one vertical for a depth-integrated sample. NOTE: When suspending a weighted-bottle sampler, use a single-filament line or rope (for example, a synthetic fiber such as nylon or Kevlar). Attach the line to the corner of the sampler to hold the bottle at a slight angle to avoid dripping river water from the line into the sample bottle.
SUPPORT EQUIPMENT Some of the equipment used for streamflow monitoring also is used as support equipment for collecting water samples. A discussion of the various types of support equipment is presented in a report by Rantz and others (1982). Great care is needed when using multipurpose equipment for water-quality sampling and sample processing. The clean hands/dirty hands technique outlined in Horowitz and others (1994) and OWQ technical memorandum 94.09 (appendix A) should be followed when using metal support equipment. With this procedure one person (dirty hands) operates the support equipment and another person (clean hands) handles the cleaned collecting equipment. Many field vehicles are used for more than one purpose (that is, streamflow measurements, gage maintenance, construction, stream sampling, and sample processing). Sample contamination is more likely to occur when multiuse vehicles are used to collect and process water samples. Therefore, it is strongly recommended that all water-quality sampling and processing be restricted to vehicles designed for that purpose. The processing area in the vehicle needs to be free of contaminants, metallic objects, dirt, and oil residue. Separate storage areas for the sampling equipment, acids, and solvents should be available, and the vehicle must be well ventilated. Several specially designed vehicles are currently in use. One example is a truck-mounted laboratory designed for use at the Rocky Mountain Arsenal by USGS personnel in Denver, Colorado.
PROCESSING EQUIPMENT The equipment used to prepare and preserve the stream samples for laboratory analyses is specific to the desired results and includes processing chambers, splitters, filtering systems, and preservation chambers. A complete list of processing equipment and supplies is given in table 3.
PROCESSING AND PRESERVATION CHAMBERS The use of processing chambers reduces the possibility of contamination and is required during the splitting and filtration processes (see Horowitz and others, 1994 and OWQ technical memorandum 94.09, appendix A). Sample preservation must be done inside separate chambers to avoid cross contamination. These processing and preservation chambers are handmade (plans are available from HIF). Generally a 2- by 2- by 2-ft frame is constructed using 1/4-in. polyvinyl chloride (PVC) to support a clear plastic bag, which forms a protective tent to work inside when processing and preserving samples.
SAMPLE SPLITTERS The cone (decaport) splitter is a positive pour-through device that composites and splits the sample in one step. It is the only splitter that has been approved for compositing and splitting samples (see Capel and Larson, 1996). A funnel-shaped reservoir receives the sample and directs it into a splitting chamber. The splitting chamber is a solid block with 10 outlet ports (placed at 36 intervals around the circumference and drilled at 45 angles) that meet in the center to form an inverted cone. The resulting configuration splits samples into 10 equal subsamples. Tests have shown that the cone splitter can split sample volumes as small as 250-mL into 10 equal subsamples, each subsample volume within an accuracy of 5 percent (see OWQ technical memorandum 80.17, appendix A). Tests of the distribution characteristics of the cone splitter (Capel and Nacionales, 1993 and Capel and others, 1995) indicate that, even with a slight difference in the volume of the subsamples, the relative percent of sediment mass to sample volumes are within 3 percent at each port, and the particle-size distribution of the finer than coarse-sand fraction is within 5 percent.
Tests indicate that the USGS churn does not produce equivalent subsamples for sediments coarser than 63 mm (Capel and Larson, 1996). There is concern that a metal spring in the spigot may contaminate the samples for trace-element analyses (see OWQ technical memorandum 94.09, appendix A). The churn should be used only as a compositing vessel for dissolved inorganic samples withdrawn from the top (see OWQ technical memorandum 94.13, appendix A). The churn is limited in sample volume and currently is available only in a plastic version.
Based on all available information, the Telfon cone splitter is the best available equipment for compositing and splitting whole water samples for analyses of major ions, nutrients, trace elements, pesticides, and sediment (see OWQ technical memorandum 96.XX, appendex A). It is presently the only alternative for splitting pesticide and sediment samples. However, when methanol is used for cleaning the cone, it is not suitable for splitting samples for total organic compounds (TOC), dissolved organic compounds (DOC), and volatile organic compounds (VOC). Those samples must be collected separately, directly from the stream, to avoid contamination. The churn is suitable for compositing dissolved inorganic constituent samples, but NAWQA studies seldom sample for these constituents in isolation. Thus, for the multipurpose needs of NAWQA, the use of the Teflon cone splitter is required.
FILTER SYSTEMS Some samples collected for inorganic constituents and most samples for organic constituents must be filtered in the field. Filtration equipment and procedures vary slightly depending on the type of constituents the filtration process is intended to isolate. The equipment basically consists of a variable-speed, battery-operated pump fitted with a peristaltic pump head or a metering pump that forces the sample through Tygon, silicon, or Teflon tubing into a filter assembly. A capsule filter system with an effective pore size of 0.45 um is used for filtering inorganic constituents. The filter type used to process the dissolved organic-carbon samples has the same pore size, but uses a stainless-steel pressure filter unit to hold a 47-mm-diameter silver filter. The plate filter used for organic-compound analyses is 142 mm in diameter and is made of glass fiber with a pore size of 0.7 um.
EQUIPMENT CLEANING
INORGANIC CONSTITUENTS AND ORGANIC COMPOUNDS
Field Cleaning
NOTE: FOR EQUIPMENT USED EXCLUSIVELY FOR ORGANIC-COMPOUND PROCESSING OMIT STEPS 3 AND 4.
Rinse with a 1-Liter solution of 5.0-percent hydrochloric
acid. The used acid/water solution should be placed in a waste
container for proper disposal (see OWQ technical memorandum 94.06,
appendix A).
Change gloves and rinse three times with DIW water.
NOTE: IF ORGANIC-COMPOUNDS SAMPLES ARE NOT COLLECTED, OMIT STEPS 5 AND 6.
Rinse the equipment used for the collection of samples for
organic-compound analyses with a minimum amount of methanol. The
used methanol should be placed in a waste container for proper
disposal (see OWQ technical memorandum 94.07, appendix A).
Allow to air dry.
Protect areas of the equipment that will contact the sample
with Teflon tape and place in a sealable plastic bag for storage and
transport.
Rinse sampling and splitting equipment at the site with 2 to 3
L of native water before sampling.
Laboratory Cleaning: When the equipment is new, has been exposed to a contaminated site, or upon inspection appears dirty, a more thorough cleaning is required. The collecting and processing equipment are disassembled, soaked in dilute phosphate-free detergent solution, rinsed with tap water, soaked in 5.0 percent hydrochloric acid (HCl), rinsed with deionized water (DIW), rinsed with methanol, and then air dried trip and between sites (see Horowitz and others ,1994 or OWQ technical memorandum 94.09, appendix A).
NOTE: FOR EQUIPMENT USED EXCLUSIVELY FOR ORGANIC-COMPOUND PROCESSING OMIT STEPS 4 AND 5.
Soak for 30 minutes in a solution of 5.0-percent hydrochloric
acid. Swirling the equipment in the acid solution will adequately desorb
any metals not removed during the washing process. The used
acid/water solution should be placed in a waste container for proper
disposal (see OWQ technical memorandum 94.06, appendix A).
Change gloves and rinse three times with DIW water.
NOTE: IF ORGANIC-COMPOUNDS SAMPLES ARE NOT COLLECTED, OMIT STEPS 6 AND 7.
Rinse the equipment used for the collection of samples for
organic-compound analyses with a minimum amount of methanol. The
used methanol should be placed in a waste container for proper
disposal (see OWQ technical memorandum 94.07, appendix A).
Allow to air dry.
Protect areas of the equipment that will contact the sample
with Teflon tape and place in a sealable plastic bag for storage and
transport.
Rinse sampling and splitting equipment at the site with 2 to 3
L of native water before sampling.
Detergents, methanol, and acids should be used with care to avoid possible contamination of the sample by their residue. A thorough native-water rinse is required at each field site before sampling to remove any remaining cleaning agents and equilibrate the equipment to the sampling conditions. A list of the supplies needed for equipment cleaning is given in table 3, and details on procedures are outlined below.
ORGANIC CARBON Equipment used for filtering the organic-carbon samples should be baked at 450 C for 2 hours or cleaned using organic-free DIW and aggressive scrubbing. USE NO DETERGENT OR METHANOL as routine cleaning agents. Protect and keep equipment away from any procedure using methanol (even the vapors could contaminate the equipment). If this equipment is contaminated and requires additional cleaning, scrub with a 0.2-percent solution of phosphate free detergent, then soak and rinse several times with large volumes of organic-free DIW.
COLLECTION METHODS Proper sampling techniques must be used to ensure that a sample is representative of the flow in the cross section. A discussion of sampling techniques is presented in reports by Edwards and Glysson (1988) and Ward and Hair (1990). Some aspects of sampling also are included in other USGS TWRIs, OWQ technical memorandums (see list of references, appendix A), and in the recommended methods for water- data acquisition (U.S. Geological Survey, 1978). A discharge measurement should be made prior to sampling if a rated discharge is not available.
Collect samples at the same cross section throughout the period of record, if possible. This will eliminate many of the potential problems that might arise during the interpretation of water-quality data. For example, measuring streamflow in a pool and sampling in a nearby riffle might prevent use of the hydraulic information to compute constituent transport. Sand may move through the pool as bedload and through the riffle as suspended load. This does not mean that the same section used during the low-water wading stage must be used during higher stages that require the use of a bridge or cableway. However, the flow characteristics at the different cross sections can result in incomparable data if the cross sections are not located near each other or in the same flow regime.
The number of verticals sampled at a site should be based primarily on the requirement to collect a sample representative of cross-sectional chemistry and secondarily to obtain the volume of the sample required. Samples usually should be collected using a standard multivertical depth-integrating method to obtain the most representative isokinetic sample possible. However, abbreviated sampling methods (that is, weighted-bottle or dip sample) are sometimes the best procedures for collecting a sample representative of the stream chemistry. Single vertical, dip, or other point-sampling methods can be used when the cross-sectional transport characteristics of the site are documented adequately or extreme flow conditions exist that preclude the use of standard methods. The Telfon bag sample might not provide a sample representative of stream hydrodynamics; however, it can collect a representative noncontaminated sample in deep or fast moving streams. Considering the limits of the other samplers, the D-77 TM might be the most appropriate sampler under many conditions, even when used beyond its limits. All samples collected by nonstandard methods should be checked periodically against standard cross-sectional samples to develop correction coefficients for the data.
Prior to initial sampling at a site, and again 3 to 4 times per year, obtain a stream profile of field measurements (velocities, specific conductance, temperature, pH, and dissolved oxygen). Record observations from several verticals and depths in the cross section to determine the uniformity of these characteristics. These measurements should be used as guides in selecting an adequate number of verticals for obtaining a representative sample.
The vertical transit rate and operational depth of each sampler is a function of the stream velocity, sample-container volume, and nozzle size. The following chart gives the recommended vertical transit rates and the maximum depths for isokinetic sampling based on samplers and nozzles. Specific limitations of the samplers are in OSW technical memorandum 94.05 (see appendix A).
_____________________________________________________________
Nozzle
Sampler diameter Ratio Depth
(inches) (feet)
_____________________________________________________________
DH-81 1/4 0.4 9
DH-81 5/16 0.4 6
D-77 TM 1/4 0.1 15
D-77 TM 5/16 0.2 15
_____________________________________________________________
Intermittent streams require special consideration because little opportunity exists to study conditions or sample in detail prior to a flow event. Rapidly changing stage, discharge, and constituent concentrations dictate that abbreviated sampling schemes and techniques be planned carefully in advance to ensure that the most representative samples possible are obtained.
Figure 1. Schematic of suggested water-quality samplers for
various stream regimes.
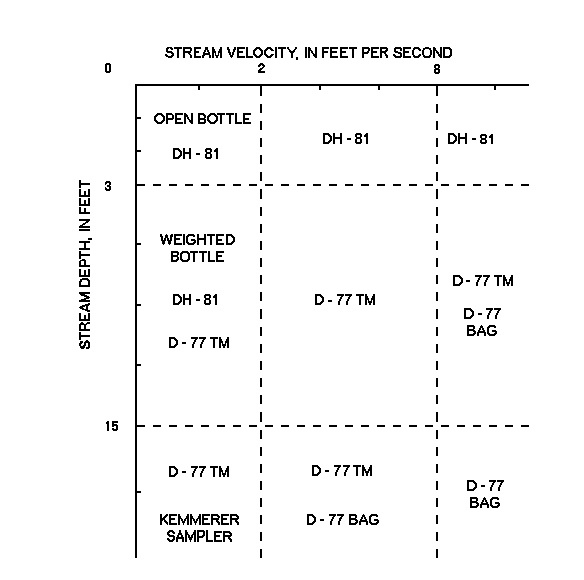
EQUAL-WIDTH-INCREMENT SAMPLING The equal-width-increment (EWI) sampling method is the recommended procedure for NAWQA. The EWI method must be used during any sampling condition where a discharge measurement is not made before sampling; where the period of discharge record is insufficient to develop stage-discharge rating curves; where the streambed material is mobile, resulting in a poor stage-discharge relationship; or where inflow from a tributary is not well mixed in the sampling section. Detailed information on EWI sampling is presented in a report by Edwards and Glysson (1988).
The EWI method requires equal spacing of a number of verticals across the cross section and an equal transit rate, both upward and downward, in all verticals. The stream width is divided into a number of equal-width intervals; the number of intervals is dependent on results of water-quality profiles, uniformity of sediment distribution, channel width, and the depth and velocity distribution across the stream. Use 5 to 10 increments for cross sections less than 5 ft wide and a minimum of 10 increments in streams 5 ft wide or greater. A maximum of 20 increments should be used in extremely wide, shallow cross sections. The sample verticals should be spaced at least 6 in. apart.
Samples from several verticals can be accumulated in the same bottle. Do not allow the bottle to overfill because secondary circulation and enrichment of heavy particles can occur and bias the sample. Empty the bottle and resample the EWI stations if overfilling should occur.
The same transit rate must be used for all verticals. When additional verticals cannot be sampled without overfilling the bottle, empty the bottle directly into the cone splitter or use another bottle and continue sampling in the same manner until all of the verticals have been sampled. Transverse the EWI verticals as many times as necessary to ensure collection of the volume of sample required for analysis. When more than one traverse of each vertical is required, the composited cross-sectional sample will be proportional to the flow if each EWI vertical is transversed an equal number of times.
EQUAL-DISCHARGE-INCREMENT SAMPLING The equal-discharge-increment (EDI) sampling method can be used on large streams only if the streamflow distribution within the cross section is known; that is, a discharge measurement is made prior to sampling. This method is not preferred, however, because it limits the number of verticals and could misrepresent a stream with stratified chemical characteristics. A discussion of this method is in a report by Edwards and Glysson (1988).
NONSTANDARD SAMPLING Most samples collected for NAWQA are obtained by the depth-integrating samplers, DH-81 and D-77 TM. The quality of samples collected using nonstandard methods is likely to be inferior to those obtained with depth-integrating samplers (see the "Sampling Equipment" section). Identify all instances of nonstandard sampling in the field notes. Below are instances where other samplers or methods might be needed.
ORGANIC-CARBON SAMPLING Special care must be taken when collecting samples for organic carbon analyses because the use of methanol as a cleaning agent will contaminate the DOC sample. Collect the sample directly into a baked 250-mL amber glass bottle using a weighted-bottle or open-bottle sampler at a single midstream vertical to avoid contamination from equipment or cleaning procedures. Because the sample probably will be more representative if the entire vertical is sampled, lower the sampler into the stream as quickly as possible. This compromised nonisokinetic collection procedure, designed to prevent equipment contamination, could affect the integrity of the suspended-organic-carbon (SOC) sample because the suspended sediments are not represented correctly.
LOW-FLOW SAMPLING In very shallow or low-flow water where a depth-integrating sampler cannot be submerged, a representative sample usually can be obtained by immersing a hand-held open bottle (dip sample) in the centroid of flow or at multiple verticals with the mouth of the bottle directed toward the current. A dip sample should never be taken when it is possible to obtain depth-integrated samples. In natural streams when velocity is greater than 1.5 ft/s, suspended sediment normally has a higher concentration near the streambed than near the surface. The bias introduced by dip sampling can be considerable if the sample is analyzed for trace elements or other constituents that can sorb onto sediment particles.
HIGH-FLOW SAMPLING If the velocity of the stream is so great that the sampler is pulled downstream and cannot be lowered in the vertical or the combination of depth and velocity cause the sampler to overfill, alternate sampling methods are necessary. Under these conditions, sample with a D-77 bag sampler or exceed the limit of D-77 TM sampler and document the procedure. The number of sampling verticals should be kept to a minimum during periods of storm runoff when the stage is rapidly changing and it is necessary to collect a large number of samples from several locations within a relatively short period of time. Under these conditions, collect the samples at a reduced number of verticals at each site and document the circumstances and number of verticals on the field notes.
SAMPLING FROZEN STREAMS During periods of extreme cold when nozzles or air exhausts in samplers freeze up, use a sampler designed for collection under ice (such as described under section, "Sampling Equipment") or collect directly into an open bottle through a hole in the ice.
AUTOMATIC SAMPLERS Automatic pumping samplers with a single-fixed intake are sometimes used to collect samples at remote sites or small streams with flashy hydrologic response. Pumped samples must be compared to EWI samples collected over the range of flow conditions at the site. EWI samples are used to develop coefficients for the point samples collected by the automatic pumping sampler. Analyze comparison samples for the same constituents as the pumped samples to determine the relation between the constituent concentrations at the single fixed-intake location and their respective mean concentrations in the cross section. Use this information to select the best location in the channel for the pump intake. Retrieve samples from the automatic sampler at the earliest possible time to reduce the chance of chemical or biological alteration of the sample. Refrigerated Teflon automatic samplers are available to help maintain sample integrity. Flow-composite samples can be obtained by withdrawing a small aliquot of sample from each bottle collected during an event and compositing into a single bottle for analyses.
SAMPLE PROCESSING The EWI sampling method produces a composite sample that is representative of flow in a cross section. When sampling for multiple chemical constituents, the sample must be subdivided within a short time after collection into a number of subsamples, each equivalent in concentration of suspended and dissolved constituents. A complete list of the equipment and supplies used in processing water samples (splitting, filtration, and preservation) is given in table 3.
Precautions must be taken to avoid contamination from the atmosphere during the processing procedure. Sample processing equipment should be kept covered (when not dispensing sample), and subsample bottles should be covered or capped. All sample preparation and processing should be done in a field processing chamber or inside a clean field vehicle.
SPLITTING The cone splitter is being used as the primary splitter to divide the collected sample into subsamples for inorganic-constituent, organic-constituent, and suspended-sediment analyses. Subsamples for filtered inorganic-constituent, organic-compound, suspended-sediment, and field analyses should be collected from the first set of split samples from the cone splitter. Subsequent splits should be used to collect subsamples for raw (unfiltered) inorganic-constituent analyses. If samples are collected only for dissolved inorganic- constituent analyses, the churn splitter may be used instead of the cone splitter (see OWQ technical memorandum 94.09, appendix A). The plastic churn splitter should not be used for compositing or splitting samples for the analysis of suspended sediment or organic compounds.
Individual samples collected in a D-77 TM sampler can be poured directly into the cone splitter from each vertical, or each time the D-77 bottle is full. Alternatively, the entire sample can be collected in several (three or four) D-77 sample bottles and later poured into the cone splitter. Either method allows the cone splitter to function as both a splitter and compositor. The sample should be well mixed in the D-77 sample bottle when dispensing the composited sample into the cone splitter. Agitate the bottle to resuspend adequately the sediment and pour rapidly into the splitter. Make sure all sediment is removed.
The splitting process is as follows:
2,000 mL - FU, pesticide analyses
250 mL - RU, laboratory conductance, pH, and alkalinity
500 mL - FU, major anions and dissolved solids
250 mL - FA, major cations
125 mL - RC, total nutrients
125 mL - FC, dissolved nutrients
250 mL - FU, field alkalinity
500 mL - RU, field measurements (conductance and pH)
1,000 mL - RU, suspended-sediment analyses
________
5,000 mL
where; F = filtered,
U = untreated,
A = acidified,
R = raw (unfiltered),
C = chilled
One 1-L bottle under each of the splitter ports will generate a set of subsamples with 800 to 900 mL in
each bottle. If additional volume is desired in the 1-L subsamples, pore one of the subsamples back
through the splitter to top off the volume in the remaining 9-L subsamples with an additional 80 to 90 mL.
Set aside three of the 1-L bottles for filtering the pesticide samples and save one 1-L bottle for
suspended-sediment analyses. Use one 1-L bottle for field measurements of conductance and pH. Two
1-L bottles can be used to filter samples for major ions, nutrients, and field alkalinity. Place a 125-mL
bottle for the nutrient sample (RC) under one splitter port and combine two ports into a 250-mL bottle
for the RU sample. Pour one of the 1-L subsamples from the first split through the splitter to fill the
second set of subsamples with the approximate volumes. The remaining two 1-L bottles can be used for
extra samples or for rinsing the equipment in the filtering process. An example of the first set of
subsamples (approximately 850 mL each) follows:
3 - Pesticides (to be filtered), 2 - Major ions, nutrients, and field alkalinity (to be filtered), 1 - Sediment (RU), 1 - Field measurements, conductance, and pH (RU), 1 - Resplit for 250-mL RU and 125-mL RC samples, and 2 - Extra samples.If a larger total-volume sample is required, use 2- or 3-L bottles under the ports of the cone splitter during the first split instead of the 1-L bottles. Additional cone splits may be necessary to achieve the proper volume of subsamples.
Three important issues to remember when using the cone splitter: never overfill a subsample bottle, always pour all of the sample into the splitter, and be careful not to spill any sample water when pouring.
SEDIMENT SAMPLE The sediment sample must be withdrawn from the cone splitter. This subsample should be taken from the first pour through the splitter. However, if for any reason the accuracy of the suspended sediment from a cone-split sample is questioned, a separate sample should be collected directly from the stream using the most appropriate method. The cone-split sediment sample is required, however, even if a separate in-stream sample is collected.
Suspended-sediment data (1) are used in traditional sediment-data interpretation such as computations of loads or erosion rates and (2) provide ancillary information for interpretation of chemistry data, particularly for constituents transported with sediment such as phosphorus. Data for the first purpose may come from the cone splitter or an appropriate sediment-sampling method such as an EWI- or EDI- sediment sample. Data for the second purpose must come from a cone-split subsample of the water collected for chemical analyses.
FILTRATION
INORGANIC CONSTITUENTS
Filter samples, such as FU, FC, FA, and the sample for alkalinity titration, with a capsule filter system (part OCALA-398 FLD). A peristaltic pump head with Tygon tubing or a Teflon diaphragm pump head with corrugated Teflon tubing should be used to create the pressure required to force these samples through the filter units.
The preservation of samples for DOC and SOC analyses is accomplished by the combined effects of filtration, chilling, and contact with the silver-filter membrane. Silver, which acts as a preservative, is dissolved from the silver filter during filtration. Most organisms will not pass through a 0.45-um filter.
Estimate the suspended-sediment concentration of the stream for filtration volumes, and then proceed to the appropriate section below. A stage/suspended-sediment graph of historical data will aid in estimating the concentrations.
_________________________________________________________________
Suspended-sediment SOC sample Processing
concentration volume procedure
(mg/L) (mL) (see below)
_________________________________________________________________
1 - 30 250 A
30 - 300 100 B
300 - 1,000 30 C
> - 1,000 10 D
_________________________________________________________________
A. Suspected suspended-sediment concentrations less than 30 mg/L:
D. Suspected suspended-sediment concentrations greater than 1,000 mg/L: Follow procedure C when processing samples suspected of having a suspended-sediment concentration greater than 1,000 mg/L, except use a 10-mL sample volume at step 3 for the SOC analyses. Step 11 then will require an additional 90 mL for the DOC sample.
Clean all equipment immediately after use, wrap with aluminum foil, and store in a sealed container (sealable plastic bag). Avoid working and storing in areas where methanol vapors might contaminate the equipment. The filter assembly and any other equipment (tweezers, graduated cylinder, and so forth) should be routinely cleaned ONLY with organic-free DIW accompanied by an aggressive scrubbing with a nonmetallic brush. DO NOT USE METHANOL OR DETERGENT for routine cleaning.
Regular inspection of the filter assembly is important to determine if additional cleaning is necessary. A dirty filter unit or a suspected contaminated filter unit will require additional cleaning. Scrub filter unit with a solution of 0.1-percent Liquinox and rinse with GALLONS of tap water (you MUST remove the detergent). Scrub and rinse with organic-free DIW. Remember that three 1-L rinses are more effective than one 3-L rinse. Double wrap the equipment with aluminum foil for storage.
ORGANIC COMPOUNDS Organic contaminants are manmade, synthetic compounds, many of which control insects (insecticides) and weeds (herbicides). The capillary-column gas chromatography/mass spectrometry (GCMS) method is used for pesticide analyses of organonitrogen herbicides (NWQL schedules 2001 and 2010). Samples collected for chorophenoxy-acid herbicides and carbamates (NWQL schedules 2050 and 2051) use the high-pressure liquid-chromatography (HPLC) method. These synthetic-organic compounds in stream water interact with sediment particles through sorptive processes; therefore, it is important to separate the solid phase from the sample as soon as possible after collection. Depth filters made from glass fibers with a 0.7-um pore size are used to filter samples for analysis of organic compounds because they can be precleaned with organic solvents or baked at 450 C. Depth filters have a high-loading capacity, making them more suitable for filtering the larger sample volumes (1 to 3 L) are needed for organic analysis. More detailed information on filtering samples is found in OWQ technical memorandum 91.09 (see appendix A) or in a report by Sandstrom and others (1992).
The pumping system should be either a valveless metering pump with a ceramic piston (FMI- QB-1-CSC) or a Teflon diaphragm head mounted on a 12-volt electric pump drive. The filter support should be made of aluminum, Teflon, or stainless steel with a 142-mm diameter. The filter support is connected to the pump with 1/4-in. convoluted or corrugated Teflon tubing and Teflon or stainless-steel fittings.
All equipment and components should be made of materials that will not contaminate or sorb analytes and are suitable for use with organic solvents such as ceramics, glass, fluorinated polymers (Teflon), stainless steel, or aluminum. The equipment should be precleaned with a Liquinox/tap-water solution (approximately 0.2-percent Liquinox by volume), rinsed with tap water and then with high-purity methanol, and air dried. Do not use the hydrochloric-acid cleaning step for equipment used in this procedure. The following procedures should be done in a clean workplace, free from fumes and dust. The samples processed here should be subsamples directly from the cone splitter.
Cartridge Processing Samples collected for analysis of organic compounds must be processed through a solid-phase extraction (SPE) cartridge within 4 days of collection. Schedules 2001 and 2050 require laboratory-processed SPE, and schedules 2010 and 2051 require field processed SPE. The SPE method utilizes bonded silica, packed into an extraction column, which absorbs specific organic compounds. These compounds subsequently are removed from the extraction column using a solvent. This procedure produces a small sample that is analyzed for selected compounds. This extracted sample can be stored for extended periods of time.
PRESERVATION Many of the ions normally present in natural waters change due to chemical and physical reactions, such as oxidation, reduction, precipitation, adsorption, and ion exchange, before analyses in a laboratory. Therefore, samples for many constituents must be stabilized by preservation. Some examples of preservative treatment are refrigeration to minimize chemical change caused by biologic activity and the addition of acid to prevent the precipitation of cations.
Below are some examples of bottles, caps, and treatments for various analyses.
__________________________________________________________________________
Analyses Bottle type Bottle cap Treatment
__________________________________________________________________________
Anions Clear plastic Black FU
Cations Clear plastic Clear FA
Nutrients Brown plastic Black RC,FC
Trace elements Clear plastic Clear FA
Organic compounds Brown glass Teflon lined FC
__________________________________________________________________________
F = filtered
U = untreated
A = nitric acid (HNO3)
R = raw (unfiltered)
C = chill and maintain to 4 C
Preservatives, such as nitric acid (HNO3), sulfuric acid (H2SO4),
hydrochloric acid (HCl), nitric acid/potassium dichromate (HNO3/K2Cr2O7),
sodium hydroxide (NaOH), and phosphoric acid (H3PO4), are available in
ampules from OCALA supply. Every measure should be taken to reduce the
possibility of contaminating samples and equipment during the
preservation process. A preservation chamber will assist in this
effort. Be sure the outside of the preservative ampules are clean.
Bottles that require no preservation should be set aside in the shipping
container. Do not add mercuric chloride (HgCl2) to the samples that will
be analyzed for nutrients (RC and FC). Samples collected for nutrient
analyses should be chilled only (see OWQ technical memorandum 94.16,
appendix A). The order in which the preservatives are added also should
be considered. ALWAYS WEAR PERSONAL-PROTECTIVE EQUIPMENT (GOGGLES,
GLOVES, AND APRON).
DISTRIBUTION All bottles must be clearly labeled with a waterproof marker or preprinted labels so the NWQL can sort the bottles for the appropriate analyses. The minimum information required is the site identification number, date and time, sample designation (bottle type), and schedule number or lab code as shown below:
09498500
01-31-1993 @ 1200
RA
SCH-2001 (or LC00114)
A NWQL analytical services request form needs to be included with each sample. The forms and the
instructions for completing the form are available from the NWQL. Be sure to retain the carbon copy of
the form.
Place all glass containers in padded sleeves or pack in some other suitable manner to prevent breakage during shipment. Chilled samples need an adequate amount of ice. Good results have been obtained by packing the chilled bottles in a volume of ice equal to approximately twice the volume of the chilled sample. The amount of ice necessary varies depending on the length of time in transit from field to laboratory and the time of year. Insulated water coolers from 1 to 5 gal in volume make good shipping containers if the integrity of the container is ensured by removing the spigot assembly and sealing with a silicon or epoxy sealer. Larger volumes of chilled samples can be sent in ice chests as long as maximum weight restrictions of the carrier are not exceeded. Guidelines on shipping samples are discussed in OWQ technical memorandum 95.04 (see appendix A).
Samples should be sent to the NWQL on the day collected when possible. The NWQL also prefers to have all bottles for a single sample sent in one container. However, nutrient samples must be sent in a separate container. Unchilled samples can be sent separately from the chilled samples.
The NWQL has issued the following guidelines:
Water temperature and dissolved oxygen should be measured directly from the stream, and several readings are required in the cross section to obtain a stream average. Specific conductance, pH, and alkalinity should be measured from a cone-split subsample so that these results will be from the same water matrix as the other chemical analyses. A single field meter that measures specific conductance, water temperature, pH, and dissolved oxygen directly in the stream may be used if stream profiles are performed regularly. These profiles must confirm that the direct in-stream measurements are comparable to the values from a cone-split sample.
Field water-quality instruments, support equipment, and the reagents used for analyses are listed in table 3, in reports by Fishman and Friedman (1985) Ward and Hair (1990), and in selected OWQ technical memorandums (79.10, 81.08, 81.17, 82.05, and 89.01, see appendix A).
Maintain an instrument log and review it prior to each field trip. The operation and calibration of all field instruments (including back-up meters and electrodes) should be checked to ensure that all are in good working condition.
TEMPERATURE The stream water temperature can affect density and gas solubility, and density affects the mixing of different water masses, especially seasonal stratification. Temperature also affects the rate of chemical reactions, biological activity, conductivity, dissolved oxygen, and pH.
Because of possible environmental contamination if broken, mercury-filled thermometers are not acceptable for field use (see OWQ technical memorandum 94.02, appendix A). The recommended procedure for determining field temperatures is a thermistor, an electrical device made of a solid semiconductor with a high temperature coefficient of resistivity. Thermistors can be constructed with a high sensitivity, but are subject to a variety of errors. Therefore, the calibration should be checked in the laboratory at several temperatures using an American Society for Testing and Materials (ASTM) thermometer to ensure the required accuracy. Never carry a mercury-filled ASTM thermometer in the field.
Field measurements of temperature should include both air-temperature and water-temperature readings. Air-temperature readings should be made by placing a dry thermistor in a shaded area protected from strong winds, but open to adequate air circulation. Avoid areas that may have radiant heat such as near metal walls or sides of vehicles. Allow the thermistor to equilibrate 3 to 5 minutes before recording the temperature.
Water temperatures should represent the mean temperature of the stream at the time of observation. A horizontal and vertical cross-section profile will determine the variability, if any, that exists. Streams with highly variable temperature profiles should have several readings averaged to use as the mean and those variations should be documented. Streams with a fairly uniform temperature (less than 2 C variance 95 percent of the time) generally will have one measurement that can be made and reported as the stream temperature. Make this measurement by suspending (from a weighted line) or placing a thermistor in midstream. Shade the thermistor probe to prevent erroneous readings caused by direct solar radiation. The thermistor should be immersed in the stream for a minimum of 1 minute prior to making measurements. Report all routine temperature measurements to the nearest 0.5 C. For special studies where more precision is required, verify the accuracy and report temperatures to the requested precision.
SPECIFIC CONDUCTANCE Conductance is the reciprocal of resistance in ohms and is a measure of the capacity of water or other substance to conduct an electrical current. Specific conductance is the conductance measured at 25 C and is reported in microsiemens per centimeter at 25 C. The specific conductance of water is determined by the types and quantities of dissolved substances in the water. Thus, specific conductance indicates the concentration of dissolved solids in water.
The specific conductance of water may change significantly with time because of pollution, precipitation, adsorption, ion exchange, oxidation, and reduction. Therefore, specific conductance should be measured in the field with an accurate conductivity meter. Many commercial conductivity meters are available on the market. All meters come with operating instructions, and users should be totally familiar with these instructions. The following are some important features and characteristics of a specific-conductance meter:
CALIBRATION Specific-conductance standards, 10 to 50,000 uS/cm at 25 C, are available from OCALA supply for meter calibration. Prior to every water-quality field trip and again onsite, standards should be used to calibrate the meter and to check meter calibration. Document calibration checks in the instrument log. Used standards should not be returned to the stock container.
Calibration and operating procedures vary with meter types and manufacturers. The procedures described below are generalized steps that should be followed and will apply to most meters used for field measurements:
NOTE: Switching meter calibration range will require recalibrating.
Discard check standard into a waste container and then rinse
electrode and container with DIW.
Record all calibration information in the instrument log
and on the field notes.
Measurements of specific conductance at stream sites should be made from an unfiltered subsample from the cone splitter. If a direct in-stream measurement is made, several readings are necessary (vertically and horizontally) in the cross section to determine a mean value.
Electrodes must be clean and properly operating to produce accurate results. The liquid junction also must be free flowing, and the electrolyte solution in the electrode must be at the proper level. Because of the variety of electrodes available, follow the cleaning and storing instructions provided by the manufacturer. Never wipe the pH electrode membrane with anything or store it dry (check manufacturer's instructions).
CALIBRATION Buffers used to calibrate and check pH meters are available from OCALA supply. The standard buffers have values of pH 4, 7, and 10 with a relatively high ionic strength. Two pH buffers are needed to calibrate the pH meter (4 and 7 or 7 and 10). Document calibration checks in the instrument log. Used standards should not be returned to the stock container.
Because calibration and operating procedures vary with meter types and manufacturers, the procedures described below are generalized steps that will apply to most meters used for field measurements:
NOTE: Turn pH meter to "standby" (or "off" on meters without standby) position prior to removing electrode from a solution.
Select a second buffer to bracket the expected stream pH.
Use a pH-10 buffer when expected pH is greater than 7 and a pH-4
buffer when the expected pH is less than 7. Always use a pH-4
buffer as the second buffer when titrating for alkalinity. Rinse
electrode, thermistor, and stirring bar with DIW. Rinse another
clean beaker, electrode, thermistor, and stirring bar with the
second buffer (pH 4 or 10). Pour second buffer into that container.
Allow temperature to equilibrate for a minute and then discard
buffer into a waste container.
Slope adjustment: Pour fresh pH buffer in the same beaker
holding the equipment. Measure temperature and remove thermistor.
Set meter temperature to the buffer temperature and, with the
stirrer on low, adjust slope to the value of pH buffer. (Some meters
have separate slope-adjustment knobs, whereas others use the
temperature knob. Always refer to instruction manual when
uncertain.) Discard pH buffer into a waste container.
Rinse electrode, thermistor, and stirring bar with DIW.
Repeat steps 3 and 4 to ensure that any slope adjustments did not
change the calibration adjustment. This is a check so adjustment
should not be needed. If adjustment is required, repeat the entire
calibration procedure.
Record all calibration information in the instrument log
and on the field notes.
Degasification, precipitation, and other chemical and physical reactions can cause the concentrations of bicarbonate and carbonate to change significantly within several hours or even minutes after sample collection. Consequently, field determinations of alkalinity, bicarbonate, and carbonate are needed in addition to laboratory determinations. Determine alkalinity, bicarbonate, and carbonate concentrations on a FILTERED sample. The section on filtration describes the proper method.
Several methods can be used to determine total alkalinity, bicarbonate, and carbonate. All of the methods involve titrating a water sample with a standard solution of sulfuric acid and monitoring the change in pH as the acid is added to the sample. The presence and quantity of hydroxide (if sample pH is 10.4 or greater), carbonate (sample pH is approximately 8.3 or greater), and bicarbonate (sample pH approximately 4.5 or greater) is determined by the pH of the sample and the quantity of acid added. The two methods commonly used are the fixed end-point and incremental methods. Additional information on alkalinity, bicarbonate, and carbonate determinations is presented in OWQ technical memorandum 82.05 (see appendix A) and in reports by Wood (1981) and Fishman and Friedman (1985).
FIXED END-POINT METHOD The fixed end-point method determines bicarbonate and carbonate by preselecting end points that correspond to true equivalence points under ideal conditions. Using sulfuric acid for titration, the sample pH is lowered to 8.3 for carbonate and 4.5 for bicarbonate. This method is not preferred because it is less accurate than the incremental method. Instructions on the fixed end-point method are given in a report by Sylvester and others (1990) (see appendix B).
INCREMENTAL METHOD The incremental method, which determines alkalinity, bicarbonate, and carbonate values more accurately than the fixed end-point method, is preferred by the USGS and should be used for NAWQA. Rather than assuming the equivalence points to be at pH 8.3 and 4.5, the incremental method determines the actual equivalence points by constructing a titration curve (plotting pH and the volume of sulfuric acid) and selecting the inflection point of the curve as the end point. The end point also can be determined by plotting the change in pH divided by the change in volume of sulfuric acid added, noting the maximum rate of change of pH per volume of acid added. The dilute-acid titrant (available from OCALA supply) should be added using a digital titrator. The digital titrator can dispense small quantities of acid and is preferred because the Quality Water Service Unit (OCALA) performs quality-controls on the normality of the titrant used in the digital titrator.
____________________________________________________________
Expected Sample Acid
alkalinity volume strength
(mg/L) (mL) (N)
____________________________________________________________
<20 100 0.160
20 - 50 50 0.160
50 - 150 100 1.600
>150 50 1.600
_____________________________________________________________
NOTE: IF SAMPLE pH IS LESS THAN 8.3, OMIT STEP 8.
If sample pH is greater than 8.3, add sulfuric acid by small
increments (1 to 3 digital counts at a time) until the pH of the
sample is below 8.0. Record pH and digital-counter reading after
each addition of acid. Allow 15 to 20 seconds for equilibration
between incremental additions. Sample should be gently stirred with
a magnetic stirrer or appropriate stirring device.
Titrate RAPIDLY to approximately pH 5.5. Allow 15 to 20
seconds for equilibration and record the digital-counter reading.
Add acid by small increments (1 to 3 digital units at a time)
from pH 5.5 to 4.0. Allow 15 to 20 seconds for equilibration
between incremental additions of acid and record pH and
digital-counter readings.
Construct a titration curve plotting the digital counts of
titrant (sulfuric acid) as a function of pH. The end points are the
inflection points of the curve (near 8.3 or 4.5), the points at which
the pH changes are greatest for volume of acid added. An alternate
method is to plot the rate of change of pH with the change in
digital counts to determine the inflection points as follows:
change in pH
-----------------------
change in digital count
Use the digital count at the maximum rate of pH change that
represents the appropriate end points to determine the following:
For 1.600N acid titration
Carbonate (mg/L as CO3-2) = 2.40 x DCA [50-mL sample]
= 1.20 x DCA [100-mL sample]
Bicarbonate (mg/L as HCO3-1) = 2.44 x (DCB-2 x DCA) [50-mL sample]
= 1.22 x (DCB-2 x DCA) [100-mL sample]
For 0.160N acid titration
Carbonate (mg/L as CO3-2) = 0.240 x DCA [50-mL sample]
= 0.120 x DCA [100-mL sample]
Bicarbonate (mg/L as HCO3-1) = 0.244 x (DCB-2 x DCA) [50-mL sample]
= 0.122 x (DCB-2 x DCA) [100-mL sample]
where
DCA is digital count near end point 8.3 pH and
DCB is digital count near end point 4.5 pH.
Alkalinity (mg/L as CaCO3) = [(CO3-2/30)+(HCO3-1/61)]x50
Report concentrations of alkalinity as equivalent calcium carbonate as CaCO3, in milligrams per liter, bicarbonate as HCO3-1, in milligrams per liter, and carbonate as CO3-2, in milligrams per liter, as follows: less than 1,000 mg/L, whole numbers; 1,000 mg/L and above, three significant figures.
Oxygen dissolved in stream water is derived from the air and from the oxygen given off by aquatic plants in the process of photosynthesis. The solubility of oxygen in water is dependent upon the partial pressure of oxygen in the air, the temperature of the water, and the mineral content of the water.
The field method most commonly used for measuring dissolved oxygen in water is the cathode/electrometric method, which uses a membrane-type electrode. Oxygen passes through a membrane at a rate relative to the partial pressure of oxygen outside the membrane. When oxygen diffuses through the membrane, it is rapidly consumed at the gold cathode. The consumption of oxygen causes a current to flow through the cell. The current is directly proportional to the quantity of oxygen consumed and can be converted to concentration units. The membrane is permeable to gases other than oxygen; therefore, halogens, hydrogen sulfide, sulfur dioxide, and helium will interfere with the ability of the probe to give correct readings. Several new methods for determining in-stream dissolved oxygen are equally dependable. Follow the instructions provided by the manufacturer for calibration and measurements when using other methods for determining dissolved oxygen. A more thorough discussion on the principles of the electrometric method of measuring dissolved oxygen is in a report by Wood (1981) and in OWQ technical memorandum 79.10 (see appendix A).
PROBE MAINTENANCE
Field probes commonly used are the Yellow Springs Instrument (YSI) series 5700 probes. Probe membranes should be checked for wrinkles and entrapped air bubbles. If present, replace membrane and electrolyte. Check for leakage of electrolyte around the membrane and the pressure-compensation diaphragm. The gold cathode should be glossy. Membranes will not last indefinitely; if the electrolyte evaporates and bubbles form under the membrane or the membrane becomes damaged or overgrown with biological growth, replace the membrane after flushing the reservoir with potassium-chloride (KC1) electrolyte. The membrane and electrolyte also should be replaced if meter readings become erratic or calibration is unstable. The zero reading on the meter should be rechecked after each membrane change. The method of probe preparation is as follows:
Keep a log of calibration information for each meter and probe. A number of general precautions and procedures should be followed prior to and during calibration of the dissolved-oxygen meter.
Elevation Subtract (feet) (millimeter mercury) 1,000 27 2,000 53 3,000 79 4,000 104 5,000 128 6,000 151Air-Calibration Chamber in Water The air-calibration chamber is the preferred procedure because it permits calibration of the oxygen meter at the temperature of the water in which the dissolved-oxygen content will be measured, thereby minimizing errors caused by temperature differences. The calibration chamber also is designed to allow the membrane surface of the probe to be at ambient-atmospheric pressure while in the chamber.
CAUTION: Be sure that no water can leak into the calibration chamber and no moisture remains on the membrane; this could reduce the rate of oxygen diffusion through the membrane, thereby producing erroneous results.
Place calibration chamber in the water where the
dissolved-oxygen content will be measured. Allow 3 to 5 minutes for
the temperature of the air inside the chamber to equilibrate with the
water.
Read the ambient atmospheric pressure from the barometer to
the nearest 5 mm of mercury. Recheck the REDLINE and ZERO reading
on the oxygen meter and adjust if necessary.
Measure temperature in the calibration chamber to the
nearest 1.0 C using the thermistor in the dissolved oxygen
probe.
Use table 4 to determine the dissolved-oxygen saturation
value at the measured water temperature and true atmospheric
pressure. Interpolate between readings for an accurate correction.
Select the scale (0-10 mg/L or 1-20 mg/L) for the
dissolved-oxygen measurement and adjust CALIBRATION control until
meter reads the dissolved-oxygen saturation value determined from
the oxygen-solubility tables. Recalibrate if the scale is changed.
After calibration, check the dissolved-oxygen meter and probe
in a dissolved-oxygen free solution. This can be accomplished by
inserting the probe into a sodium-sulfate/cobalt-chloride solution
and measuring the dissolved oxygen. If the instrument reading
exceeds 0.2 mg/L, the membrane and electrolyte or the entire probe
might need to be replaced. Thoroughly rinse probe with DIW to
remove any residue of the zero-dissolved-oxygen solution.
Meter is now calibrated and ready for use.
[Solubility of dissolved oxygen is in mg/L. mm/Hg, millimeter of mercury; C, degree Celsius] ____________________________________________________________________________ Water Atmospheric pressure (mm/Hg) Temp --------------------------------------------------------------------- (C) 760 740 720 700 680 660 640 620 600 580 ____________________________________________________________________________ 0.0 14.6 14.2 13.8 13.4 13.0 12.7 12.3 11.9 11.5 11.1 2.0 13.8 13.4 13.1 12.7 12.3 12.0 11.6 11.2 10.9 10.5 4.0 13.1 12.7 12.4 12.0 11.7 11.4 11.0 10.6 10.3 10.0 6.0 12.4 12.1 11.8 11.4 11.1 10.8 10.4 10.1 9.8 9.5 8.0 11.8 11.5 11.2 10.9 10.6 10.2 9.9 9.6 9.3 9.0 10.0 11.3 11.0 10.7 10.4 10.1 9.8 9.5 9.2 8.9 8.6 12.0 10.8 10.5 10.2 9.9 9.6 9.3 9.0 8.8 8.5 8.2 14.0 10.3 10.0 9.7 9.5 9.2 8.9 8.6 8.4 8.1 7.8 16.0 9.8 9.6 9.3 9.1 8.8 8.5 8.3 8.0 7.7 7.5 18.0 9.4 9.2 8.9 8.7 8.4 8.2 7.9 7.7 7.4 7.2 20.0 9.1 8.8 8.6 8.3 8.1 7.8 7.6 7.4 7.1 6.9 22.0 8.7 8.5 8.2 8.0 7.8 7.5 7.3 7.1 6.8 6.6 24.0 8.4 8.2 7.9 7.7 7.5 7.2 7.0 6.8 6.6 6.3 26.0 8.1 7.9 7.6 7.4 7.2 7.0 6.8 6.5 6.3 6.1 28.0 7.8 7.6 7.4 7.2 7.0 6.7 6.5 6.3 6.1 5.9 30.0 7.5 7.3 7.1 6.9 6.7 6.5 6.3 6.1 5.9 5.7 ____________________________________________________________________________ R.F. Weiss (1970) Deep Sea Research, v. 17, p. 721.Air-Saturated Water This method calibrates the dissolved-oxygen meter using water saturated with oxygen at a known temperature and ambient atmospheric pressure.
CAUTION: It is extremely important to ensure that the water is 100-percent saturated with oxygen. Observe meter while aerating calibration water. If no change in dissolved oxygen is observed for a period of 2 to 3 minutes, the water can be assumed to be saturated.
Read the ambient atmospheric pressure from the
barometer to the nearest 5 mm of mercury. Recheck the REDLINE and
ZERO reading on the oxygen meter and adjust if necessary.
Measure temperature in the water chamber to the nearest 0.5 C
using the thermistor in the dissolved-oxygen probe.
Determine the dissolved-oxygen saturation value at the
measured water temperature and true atmospheric pressure using table 4.
Select the scale (0-10 mg/L or 1-20 mg/L) to be used for the
dissolved-oxygen measurement and adjust CALIBRATION control until
meter reads the dissolved-oxygen saturation value determined from
table 4. Recalibrate if the scale is changed.
After calibration, check the dissolved-oxygen meter and probe
in a dissolved-oxygen-free solution. This can be accomplished by
inserting the probe into a sodium-sulfate/cobalt-chloride solution
and measuring the dissolved oxygen. If the instrument reading exceeds
0.2 mg/L, the membrane and electrolyte or the entire probe might
need to be replaced. Thoroughly rinse probe with DIW to remove any
residue of the zero-dissolved-oxygen solution.
Meter is now calibrated and ready to use.
The dissolved oxygen should be measured with the probe immersed directly in the stream. If the stream velocity at the point of measurement is less than 1 ft/s, use a stirrer or raise and lower the probe at a rate of approximately 1 ft/s (do not break the surface of the water). If the stream velocity is so high that the probe will not submerged, attach the probe to a small weight on a separate line.
measured DO (mg/L)
DO (% saturation) = ----------------------- x 100
DO at saturation (mg/L)
where DO is dissolved oxygen.
DOCUMENTATION
Field activities should be documented on standard surface-water-quality
field notes BQA-1 3/92. Figure 2 shows page 1 of the field notes. Pages
2, 3, and 4 include meter calabration information, quality assurance
samples, alkalinity titration summary, and cross-section profile
information. Complete all appropriate sections. A complete
documentation will aid in future analyses of the collected information.
Field notes should include the following information:
Figure 1. Surface-water quality field notes.
QUALITY ASSURANCE AND QUALITY CONTROL
The sources of variability and bias introduced by sample collection and processing affect the interpretation of water-quality data. Establishment of quality-assurance plans ensure that the data collected are compatible and of sufficient quality to meet program objectives. This field guide and accompanying references, along with the study-unit design guidelines for NAWQA, should be used by the study units when preparing quality-assurance plans. Specific details for quality- assurance plans are described in a report by Shampine and others (1992).
Investigators in each study unit must document the quality of their data by collecting quality-control samples. A series of quality-control samples (field blanks, replicates, and field-matrix-spike samples) is obtained in water-quality investigations (Shampine and others, 1992) because the quality of the data collected and the validity of any interpretation cannot be evaluated without quality-control data. Quality- control samples should include the same sample set as the routinely scheduled samples. For the detailed procedures for preparing quality-control samples and the required percent of samples necessary, consult the NAWQA quality-assurance memorandums cited in appendix B. Additional quality-control requirements for inorganic constituent sampling and processing are discussed in OWQ technical memorandum 94.09 (see appendix A).
FIELD BLANKS Field blanks are designed to demonstrate that (1) equipment-cleaning protocols adequately remove residual contamination from previous use, (2) sampling and sample-processing procedures do not result in contamination, and (3) equipment handling and transport between periods of sample collection do not introduce contamination.
Field blanks for pesticides are collected immediately before processing native water through the sample-processing sequence for field samples. Preparation of field blanks requires passing a volume of organic-free DIW through all sample equipment contacted by the actual sample.
Field blanks for major ions and nutrients should be collected by the same approach, but using inorganic-free DIW after preparation of the organic blank.
REPLICATES Sample replicates are designed to provide information needed to (1) estimate the precision of concentration values determined from the combined sample-processing and analytical scheme and (2) evaluate the consistency of identifying target analytes for pesticides. Each replicate sample is an aliquot of native sample water from a splitter and is processed immediately after the primary cone-split sample using the same equipment; placed into the same type of bottle; prepared in the same way by SPE, if applicable; and stored and shipped in the same way.
FIELD-MATRIX SPIKES Field-matrix spikes are designed to (1) assess recoveries from field matrices and (2) assist in evaluating the precision of results for the range of target analytes in different matrices.
A field-matrix spike is prepared by adding a standard spike solution provided by NWQL to a split of sample water processed in the same way as the regular pesticide analysis. A separate matrix-spike sample for each of the two pesticide schedules is prepared, stored, and shipped to NWQL. Matrix-spike kits with instructions are available from NWQL.
References Cited Busenberg, Eurybiadas, and Plummer, L.N., 1987, pH measurement of low-conductivity waters: U.S. Geological Survey Water-Resources Investigations Report 87-4060, 22 p.
Capel, P.D., Nacionales, F.C., and Larson, S.J., 1995, Precision of a splitting device for water samples; USGS Open File Report 95-293, 6 p.
Capel, P.D., and Larson, S.J., 1994, Evaluation of selected information on splitting devices for water samples: U.S. Geological Survey Water-Resources Investigations Report 95-4141, 103 p.
Crawford, J.K., and Luoma, S.N., 1992, Guidelines for studies of contaminants in biological tissues for the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report 92-494, 69 p.
Edwards, T.K., and Glysson, D.G., 1988, Field methods for measurement of fluvial sediment: U.S. Geological Survey Open-File Report 86-531, 118 p.
Fishman, M.J., and Friedman, L.C., eds., 1985, Methods for determination of inorganic substances in water and fluvial sediments: U.S. Geological Survey Techniques of Water-Resources Investigations, book 5, chap. A1, 545 p.
Gilliom, R.J., Alley, W.M., and Gurtz, M.E., 1994, Design of the National Water-Quality Assessment program: Occurrence and distribution assessment: U.S. Geological Survey Circular 1112, 33 p.
Hem, J.D., 1985, Study and interpretation of chemical characteristics of natural water: U.S. Geological Survey Water-Supply Paper 2254, 264 p.
Hirsch, R.M., Alley, W.M., and Wilber, W.G., 1988, Concepts for a National Water-Quality Assessment program: U.S. Geological Survey Circular 1021, 42 p.
Horowitz, A.J., Demas, C.R., Fitzgerald, K.K., Miller, T.L., and Rickert, D.A., 1994, U.S. Geological Survey protocol for collection and processing of surface-water samples for the subsequent determination of inorganic constituents in filtered water: U.S. Geological Survey Open- File Report 94-539, 57 p.
Koterba, M., Wilde, F., and Lapham, W., 1996, Ground-water data-collection protocols and procedures for the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report 95-399, 113 p.
Leahy, P.P., Rosenshein, J.S., and Knopman, D.S., 1990, Implementation plan for the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report 90-174, 10 p.
Meador, M.R., Cuffney, T.F, and Gurtz, M.E., 1993, Methods for sampling fish communities as part of the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report 93-104, 40 p.
Rantz, S.E., and others, 1982, Measurement and computation of streamflow: Volume 1, Measurement of Stage. Volume 2, Computation of discharge: U.S. Geological Survey Water-Supply Paper 2175, v. 1, p. 1-284, v. 2, p. 285-631.
Sandstrom, M.W., Wydoski, D.S., Schroeder, M.P., Zamboni, J.L., and Foreman, W.T., 1992, Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory--determination of organonitrogen herbicides in water by solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring: U.S. Geological Survey Open-File Report 91-519, 19 p.
Shelton, L.R., and Capel, P.D., 1994, Guidelines for collecting and processing samples of stream bed sediment for analysis of trace elements and organic contaminants for the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report 94-458, 20 p.
Shelton, L.R., Field guide for collecting volatile organic compounds in stream water for the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report in progress, 11 p.
Shampine, W.J., Pope, L.M., and Koterba, M.T., 1992, Integrating quality assurance in project work plans of the U.S. Geological Survey: U.S. Geological Survey Open-File Report 92-162, 12 p.
U.S. Geological Survey, 1978, Sediment, chapter 3 in U.S. Geological Survey, National handbook of recommended methods for water-data acquisition: p. 3-1 to 3-100.
Ward, J.R., and Hair, C.A., 1990, Methods for collection and processing of surface-water and bed-material samples for physical and chemical analyses: U.S. Geological Survey Open-File Report 90-140, 71 p.
Weiss, R.F., 1970, The solubility of nitrogen, oxygen, and argon in water and seawater: Deep Sea Research, v. 17, no. 4, p. 721-735.
Wood, W.W., 1981, Guidelines for collection and field analysis of ground-water samples for selected unstable constituents: U.S. Geological Survey Techniques of Water-Resources Investigations, book 1, chap. D2, 24 p.
APPENDIX A--SELECTED TECHNICAL MEMORANDUMS
These Office of Water Quality (OWQ), Office of Surface Water (OSW) and
Water Resources Division (WRD) memorandums are available in U.S.
Geological Survey, Water Resources Division offices, nationwide.
OWQ 79.10 ANALYTICAL METHODS Recommended procedures for calibrating
dissolved oxygn meters
OWQ 80.17 EQUIPMENT AND SUPPLIES New sample splitter for water-quality
samples
OWQ 81.02 WATER QUALITY Operation and availability - D-77
water-quality sampler
OWQ 81.08 WATER QUALITY Electrodes for pH measurements in
low-conductivity waters
OWQ 81.17 EQUIPMENT AND SUPPLIES YSI model 32 conductance meters
OWQ 82.05 WATER QUALITY Method for dissolved carbonate,
dissolved bicarbonate, and carbonate
alkalinity
OWQ 89.01 EQUIPMENT AND SUPPLIES pH measurement in low conductivity waters
OWQ 90.01 WATER QUALITY Sample preservation and ampule disposal
OWQ 91.02 PUBLICATIONS Methods for collection and processing
of surface-water and bed-material
samples for physical and chemical analyses
OWQ 91.09 REPORTS Filtration of water-sediment samples
for the determination of organic compounds
OWQ 91.10 PROGRAMS AND PLANS Dissolved trace element data
(contamination)
OWQ 92.01 PROGRAMS AND PLANS Dissolved/deionized water for district
operations
OWQ 92.02 FIELD TECHNIQUES Field preparation of containers for
aqueous samples
OWQ 92.11 FIELD TECHNIQUES Return of spent mercury and dichromate
ampules to the national water quality
laboratory
OWQ 92.12 PROGRAMS AND PLANS Trace element concentrations in
deionized water processed through
selected surface-water samplers
OWQ 92.13 PROGRAMS AND PLANS Trace element contamination: findings
of studies on the cleaning of
membrane filters and filtration systems
OWQ 93.05 PROGRAMS AND PLANS Evaluation of capsule filters
OWQ 93.06 PROGRAMS AND PLANS Trace element contamination--findings
of study on the cleaning of sampler
caps, nozzles, bottles, and bags
OWQ 93.11 PROGRAMS AND PLANS Protocol for collecting and processing
surface-water samples for low-level
inorganic analyses
OWQ 94.02 EQUIPMENT Discontinuance of field use of mercury
liquid-in-glass thermometers
OSW 94.05 EQUIPMENT Maximum sampling depths and transit
rates for suspended sediment and
water-quality samplers
WRD 94.06 SAFETY Storage, transport, handling, and
disposal of hydrochloric acid
WRD 94.07 SAFETY Storage, transport, handling and
disposal of methyl alcohol
OWQ 94.09 PROGRAMS AND PLANS Revision of protocol for collecting
and processing surface-water samples for
low-level inorganic analyses.
OWQ 94.13 EQUIPMENT Evaluation of churn splitter for
collection and processing surface-water
samples for determination of trace
elements, nutrients, and major ions in
filtered water.
OWQ 94.16 PROGRAMS AND PLANS New preservation techniques for
nutrient samples.
OWQ 95.04 FIELD TECHNIQUES Shipping samples to the National Water
Quality Laboratory.
OWQ 96.XX FIELD TECHNIQUES Protocol for cleaning and using the
Teflon cone splitter to produce
noncontaminated subsamples for subsequent
determinations of trace elements in
filter water.
APPENDIX B--SELECTED INTERNAL COMMUNICATIONS
These documents are available in U.S. Geological Survey, Water Resources
Division offices where the National Water-Quality Assessment Program have
active studies.
National Water-Quality Assessment program, written communication, U.S. Geological Survey memorandum dated June, 1996, Quality-Control Objectives and Procedures for NAWQA Surface-Water Sites.
Sylvester, M.A., Kister, L.R., and Garrett, W.B., eds, 1990, Guidelines for collection, treatment, and analyses of water samples--U.S. Geological Survey Western Region Field Manual: U.S. Geological Survey, Western Region, Internal Document, 144 p.