
This document is also available in pdf format:
![]() fs-005-97.pdf
fs-005-97.pdf
Acidic drainage from mines has been recognized as a problem since mining began in this country over a century ago. The U.S. Forest Service has estimated that more than 1,500 mining sites in the western United States contribute to the problem. Other estimates have placed the total number of mine sites involved at 20,000 to 50,000. In many instances, acidic drainage, which consists of flows or seeps into streams and rivers, can adversely affect aquatic ecosystems and degrade drinking-water quality. In order to remediate the worst abandoned mine sites and minimize the effect from existing and new mines, adequate knowledge is needed of the physical and chemical processes that control the movement and fate of contaminants.
The U.S. Geological Survey (USGS) began research on acidic metal contamination in the Pinal Creek Basin in 1984. Research has been funded mainly by the USGS Toxic Substances Hydrology Program, the goal of which is to provide earth-science information on the behavior of toxic substances in the Nation’s surface waters and ground waters. The program does this through research on the processes that control contaminant movement and through the development of new methods with which to undertake this research. USGS research in Pinal Creek Basin initially focused on a plume of acidic ground water in the regional alluvium, but has broadened to include investigations of streamflow contamination and transport interactions between streamflow and shallow ground water. Research at the site is now a multidisciplinary, collaborative effort among scientists from the USGS, University of Arizona, and Arizona State University. The overall objectives are to increase scientific understanding of the understanding of the controls on transport of metals and other inorganic contaminants and to develop new methods and models to investigate contaminant transport.
Pinal Creek Basin is in central Arizona about 100 kilometers east of Phoenix (fig.1). Globe and Miami are the principal communities in the basin, which had a population of about 18,000 in 1990. Copper has been mined in the basin since 1882—first in underground mines and, beginning in 1948, in openpit mines. The largest open-pit mines in the basin are adjacent to and north of Miami, where mines and tailings dominate the local landscape (fig. 2).
In the late 1980’s, Pinal Creek Basin was placed in the Water Quality Revolving Fund (WQARF), which is the State of Arizon’s equivalent of the U.S. Environmental Protection Agency (USEPA) Superfund Program. At about the same time, three mining companies currently or formerly active in the basin formed the “Pinal Creek Group” to reduce contaminant sources and remediate existing contamination. Information from the USGS studies was used by the Pinal Creek Group to help develop a cost - effective plan for remediation of the aquifer.
Russell Gulch and Bloody Tanks Wash combine to form Miami Wash, which is the main tributary to Pinal Creek in the basin. These and other tributaries drain the hills and mountains that surround the valley occupied by Pinal Creek and Mi ami Wa sh. Unconsolidated stream alluvium and underlying basin fill form the regional aquifer in Pinal Creek Basin. The alluvium is from 300 to 800 meters wide, less than 50 meters thick, and contains poorly sorted, cobble- to claysized material. Most material, however, is sand size or greater. The regional aquifer is bounded laterally and at depth by older, less permeable rocks that include granite, schist, and volcanic rocks. Infiltration of intermittent storm runoff and snowmelt from surrounding mountains through permeable streambed alluvium in the basin is a major source of recharge to the aquifer. In the northern part of Pinal Creek Basin, the aquifer becomes shallower in the direction of groundcalcium, water flow and Inspiration Dam (fig. 3). As a result, ground water flows upward to the stream channel of Pinal Creek and forms perennial st reamflow for approximately 7 kilometers from near Horseshoe Bend Wash to Inspiration Dam. Past Inspiration Dam, Pinal Creek flows through a narrow rock-walled canyon to the Salt River and eventually to Roosevelt Lake, an important water - supply reservoir for the City of Phoenix.
 |
| Figure 3. Perennial streamflow in Pinal Creek, which consists of a mixture of contaminated and uncontaminated ground water that has discharged to the surface, flows over Inspiration Dam and enters the Salt River just above Roosevelt Lake. |
In Pinal Creek Basin, acidic mine drainage over the past century has resulted from oxidation of sulfide minerals, from ore processing, and from other mining activities in the basin. Unlined surface-water impoundments of acidic water were significant contaminant sources. The single largest source probably was Webster Lake, which existed from about 1940 to 1988. Available data indicate that from the mid-1970’s until the lake was drained in 1988, pH of the lake water was between 2 and 3. Concentrations of dissolved iron and sulfate were greater than 2,000 and 19,000 milligrams per liter, respectively.
Contaminated acidic ground water was first recognized in the regional aquifer in the 1930's, and perennial streamflow in Pinal Creek is known to have been affected by contamination since 1963 (Envirologic Systems, Inc., 1983) and possibly earlier. A plume of contaminated ground water has moved about 25 kilometers through the stream alluvium of Miami Wash and Pinal Creek. The acidic part of the plume contains large concentrations of sulfate, groundcalcium, iron, manganese, copper, aluminum, and zinc. In 1996, concentrations of dissolved iron were 200 milligrams per liter at well 51 and 190 milligrams per liter at well 402.
Acidic ground water is neutralized mainly through reaction with calcium carbonate as the plume moves downgradient. The pH increases to between 5 and 6, and metals including iron, copper, and zinc precipitate or adsorb to mineral surfaces in the aquifer. Manganese dissolves and becomes mobile in ground water. As ground water surfaces in the perennial reach of Pinal Creek, the pH of contaminated stream water increases to about 8 as the water equilibrates with the atmosphere and thus causes manganese carbonate and oxide minerals to precipitate as crusts in the streambed.
Major changes in the chemistry of the ground-water plume have occurred because of the removal of the major source of contaminants, withdrawal of contaminated ground water, dilution from natural recharge, better control of surface runoff, and subsurface chemical reactions. Concentrations of dissolved iron in water from well 51 decreased from 3,200 milligrams per liter in November 1984 to 1,700 milligrams per liter in November 1990. During that time, ground-water levels declined about 16 meters despite normal amounts of rainfall in most years. Greater - than- average winter precipitation caused water levels to rise as much as 16 meters from January 1991 to May 1993. By June 1994, concentrations of dissolved iron in well 51 had decreased to 200 milligrams per liter (fig. 4).
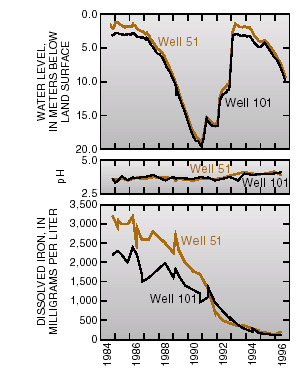 |
| Figure 4. Ground-water levels, pH, and concentrations of iron in ground water adjacent to Miami Wash, 1984–96. |
Two general themes have guided research at the site. The first is the development of new tools to assess the transport and fate of contaminants in the stream- aquifer system. The tools and techniques developed at Pinal Creek will be transferable to similar sites affected by acidic mine drainage.
The second theme is the evaluation of computer models to characterize the physical and chemical processes that occur at contaminant sites, to help design experiments, and to assess the effectiveness of remediation at Pinal Creek Basin and elsewhere. A valuable component of any modeling exercise is uncertainty analysis. Modeling and uncertainty analysis are necessary to generalize research results and transfer data to other areas affected by mining.
Research in Pinal Creek Basin has determined that:
—Dissolved-metal contaminants in the plume move much more slowly than the plume water. Neutralization reactions in the carbonate aquifer slow the rate of advance of the plume's acidic front and most of the dissolved metals to about one seventh of the rate of advective ground-water flow.
—The oxidation and precipitation of iron is driven by the reductive dissolution of manganese oxide minerals. This reaction is a source of additional acidity to the plume and a source of dissolved manganese, which travels ahead of the plume’s acidic front in neutralized ground water.
—The downgradient decrease in dissolved concentrations of copper, cobalt, nickel, and zinc was the result of the pH- dependent adsorption to iron hydroxides in the aquifer.
>—Geochemical modeling has revealed the need for information on redoxreaction rates in the plume and on carbon dioxide leaving the plume and oxygen entering the plume in order to better understand controls on pH and movement of contaminants.
>—The exchange of gases between the plume and atmosphere affects the geochemistry of the shallow part of the plume. The major cont rol s on exchange of carbon dioxide and oxygen from the plume across the water table are (1) neutralization reactions involving carbonate minerals and (2) oxidation of dissolved iron near the water table.
>—After contaminated ground water discharges to Pinal Creek, gas exchange causes a rapid increase in pH and dissolved oxygen in streamflow. Continual movement of perennial streamflow into and out of areas of shallow ground water beneath the stream (hyporheic zone, fig. 5) increases the contact of streamflow with sediment sur faces . The increased contact stimulates precipitation of dissolved manganese as oxide coatings on streambed sediment.
>—Chemical reactions in the hyporheic zone are enhanced by the activity of manganese oxidizing bacteria, which are active in the zone because of input of oxygenated streamflow. Approximately twenty percent of the total load of dissolved manganese flowing out of the drainage basin is removed in this manner.
Precipitation of manganese in the hyporheic zone reduces the movement of dissolved nickel and cobalt because manganese oxides are an excellent sorbent for those metals. As a result, the loads of dissolved nickel and cobalt flowing from the basin each were reduced because of hyporheic exchange.
>—Solute tracers are useful in deter - mining the extent of chemical reactions in streamflow and shallow ground-water systems. The technique, however, sometimes has high uncertainty. A new method was developed to improve the design of tracer experiments to reduce uncertainty when using tracers to estimate rates of transport and chemical reaction.
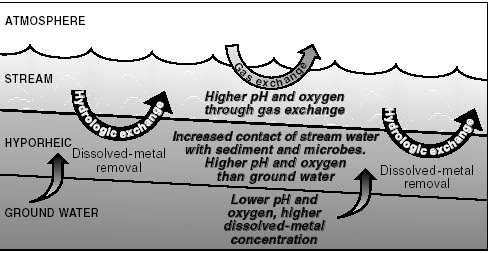 |
| Figure 5. In the perennial reach some of the stream water continually moves into and out of an area in shallow ground water known as the hyporheic zone. This process helps enhance removal of dissolved metals from streamflow. |
Research focuses on the processes in the two major hydrologic regimes at the site: ground-water transport in the southern part of the basin and interaction of surface-water and shallow groundwater transport in the northern part of the basin. Ongoing and proposed research are designed to:
—Determine the hydraul ic-flow characteristics of discrete intervals of aquifer sediments. Contaminants move preferentially along zones of higher permeability. Accordingly, the permeability of such discrete zones determines, in part, the rate at which contaminants move through the groundwater system.
—Using parameter estimation and sensitivity-analysis techniques, evaluate and determine the value of different types of data that are used in groundwater models. Constrain the flow system with ground-water ages obtained from chlorofluorocarbon analyses.
—Use geochemical model ing to determine appropriate simplifications that can be made. For example, what is the ef fect of as suming that the mineralogy of the aquifer is homogeneous? Information on appropriate simplifications will be useful at similarly contaminated sites where data are limited and may help to determine the types of data that need to be collected.
—Develop improved instrumentation and more efficient data - collection techniques to determine rates of uptake of dissolved metals in the hyporheic zone.
—Identify the most important physical and chemical processes that stimulate enhanced chemical reactions in the hyporheic zone and thus reduce the load of metal contaminants flowing out of the Pinal Creek drainage basin.
—Transfer stream-tracer design methodology to the USGS Abandoned Mine Lands Initiative. The new design strategy will improve the efficiency of future experiments and increase the precision of tracer methods as a means of characterizing contamination and chemical reactions in surface water and shallow ground water at sites with different characteristics from those of Pinal Creek.
—James G. Brown, Robert Brew, and Judson W. Harvey
Brown, J.G., and Favor, Barbara, eds., 1996, Hydrology and geochemistry of aquifer and stream contamination related to acidic water in Pinal Creek Basin near Globe, Arizona: U.S. Geological Survey Water-Supply Paper 2466, 103 p.
Gellenbeck, D.J., and Hunter, Y.R., 1994, Hydrologic data from the study of acidic contamination in the Miami Wash–Pinal Creek area, Arizona, water years 1992–93: U.S. Geological Survey Open-File Report 94–508, 103 p.
Brown, J.G., and Harvey, J.W., 1996, Hydrologic and geochemical factors affecting metal - contaminant transport in Pinal Creek basin near Globe, Arizona, in Morganwalp, D.W., and Aronson, D.A., eds., U.S. Geological Survey Toxic Substances Hydrology Program — Proceedings of the Technical Meeting, Colorado Springs, Colorado, September 20–24, 1993: U.S. Geological Survey Water- Resources Investigations Report 94 – 4015, v. 2, p. 1035–1042.
For more information contact:
or
Judson Harvey| AccessibilityFOIAPrivacyPolicies and Notices | |
 |
|