SF5CF3 Sampling
In This Document
- Equilibration of a Headspace with the Four Tracers in the Field
- Source of bottles and caps
- Steps for Field Collection of SF5CF3 and CFC-13 from Ground Water Samples
- General Comments and Limitations of the New Procedure
- Other Considerations
Equilibration of a Headspace with the Four Tracers in the Field
Because of the low concentrations of SF5CF3 and CFC-13 in ground water, normal purge-and-trap GC-ECD analytical procedures, such as those used for analysis of CFCs [Busenberg and Plummer, 1992] and SF6 [Busenberg and Plummer, 2000] would require complete extraction from tens of liters of ground water to obtain sufficient sample for analysis. Collection and transport of such large volumes of water without contamination of the sample is impractical. Therefore, in this study, the tracers were extracted from ground water in the field using a headspace-equilibration procedure [see Appendix A1 in Busenberg and Plummer, 2000]. With this procedure, approximately one hour is required for the headspace partial pressure to equal that of the gas partial pressure in the water sample.
The apparatus used with the headspace spray method is shown below.

Apparatus used to equilibrate a headspace with ground water.
It consisted of a 0.25 L graduated plastic cylinder that was placed as shown inside a 1 L cylinder. A septum valve was threaded to a small hole in the base of the 0.25 L plastic cylinder. The larger cylinder was filled with water while septum and cap were not on the valve, allowing the air in the small cylinder to escape as the large cylinder was filled with ground water. After both cylinders were filled with water, the water flow through the 8 spray nozzle tube was initiated. The water flow through the nozzles was maintained for about 3 minutes before the septum and cap were attached. Nitrogen gas was injected through the septum with a syringe forming a headspace and exposing the spray nozzles. After equilibration, the headspace gas was removed through the septum with a 60 mL syringe. The gas sample was injected into an inverted 125 mL bottle in a beaker filled with the same ground water as shown replacing most but not all the water in the bottle.

Method used to collect the headspace gas and store it without contact with air. The same procedure is used to sample the gas for analysis.
The bottle is capped underwater with an aluminum foil-lined cap. The bottle is dried and the cap is secured with electrical tape and stored upside down. In the laboratory, the tape is removed, the bottle is weighed, and placed in a beaker filled with water that has been stripped with N2. The cap is removed underwater. Stainless steel tubing filled with gas-stripped water with a septum at one end is inserted into the bottle and the gas sample is removed with a syringe. The tubing then is removed, the bottle capped underwater, dried, and then weighed again to determine the volume of gas in the bottle.
In laboratory experiments, water was sprayed at rates of 2 L/min through eight 0.5mm diameter nozzles into the headspace. The composition of the headspace gas was determined by gas chromatography as a function of time. In 10 minutes or less, equilibrium was reached between dissolved nitrogen, oxygen, and argon in the water and the headspace gas. In about 30 minutes equilibrium was reached with CFC-12 and SF6. Busenberg and Plummer [2000] evaluated the time needed to reach equilibrium with various headspace gases at various spraying rates. Equilibrium with SF5CF3 was reached in one hour, for samples near the detection limit of SF5CF3 (0.005 pptv; 1970 air). N2 or He was used as the headspace gas for relatively old samples with low concentrations of SF5CF3, however, for very young waters, air can be used as the headspace gas which reduces the equilibration time. This equilibration procedure requires spray of at least 120 L of water over a period of about 1 hour.
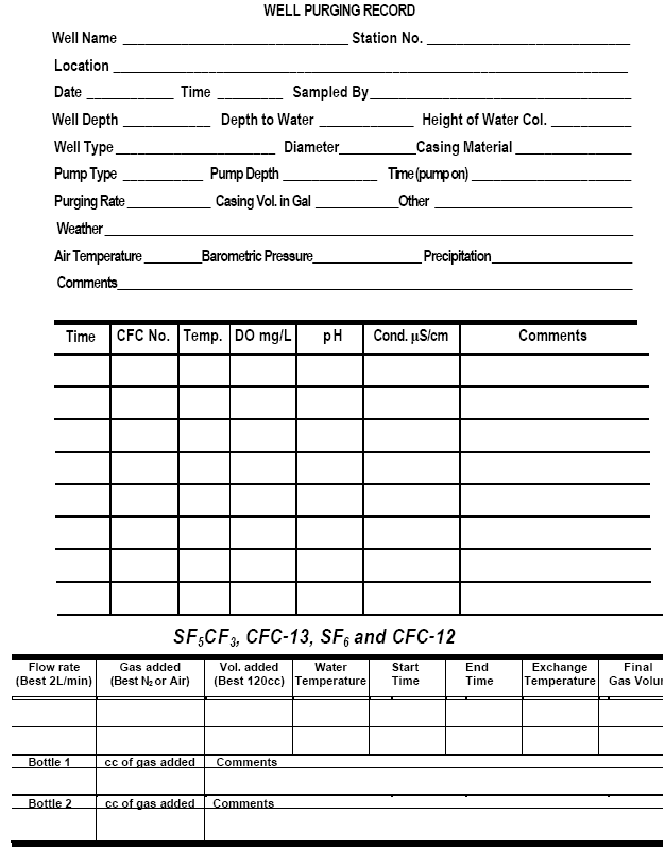
Complete one copy of this well-purging form for each ground-water sample.
Source of bottles and caps
Bottles and caps can be obtained from the Scientific Specialties Co. at 800-648-7800. The bottles are 125ml (4 oz) Boston round clear glass and have a cap size 22-400.
Item No. B73504 is a case of 24 bottles with Teflon lined caps. These bottles have the wrong caps! Discard these caps and replace them with the caps below.
Bottles are also available from any Wheaton glass supplier as Wheaton part number 217112, which is a case of 24 bottles with no caps.
The caps are sold as Scientific Specialties item no. A69522, white plastic caps with aluminum foil liner in a bag of 72. Use only these aluminum lined caps! This cap is the key to the method. Discard any caps, if the foil liner appears scratched, dented, or altered in any way.
[an error occurred while processing this directive]The bottles use are the same as those for CFCs and are are available on USGS OneStop Shopping. They are stock numbers Q604FLD for the bottles and Q603FLD for the caps.
Steps for Field Collection of SF5CF3 and CFC-13 from Ground Water Samples
- Purge well
- Connect tubing from pump to the stripping apparatus (see figure of apparatus)
- Remove the septum from the stripping cell
- Allow the water to fill the 2 liter graduated cylinder
- Measure the flow through the spray nozzles (flow must be greater than 1.7 L per minute)
- Stop the flow of water by closing the valve to the spay chamber
- Then rapidly replace the septum
- Add 120 cm3 of pure nitrogen through the septum into the spray chamber
- Record the spray rate, the temperature of the ground water and the time.
- After one hour stop the flow (100 L of water)
- Fill a 2 L beaker with well water
- Fill the 125 mL bottle with well water and insert bottle in upside down in the beaker (see figure of beaker)
- Remove the headspace gas with a syringe and inject it into the bottle as shown in figure, a little water should be left in the bottle (120 mL of gas)
- Cap the bottle underwater
- Remove the bottle from beaker and dry the bottle with a towel
- Tape the cap onto the bottle with electrical tape
- Label the bottle with the well name
- Place the bottle upside down in to the original cardboard box
- If there is additional gas in the stripping cell, the collect a second bottle using the same procedure
- Keep bottles in the cooler and not in the sun
General Comments and Limitations of the New Procedure
- The new procedure requires stripping of gases from the ground water for one hour at water flow rates of greater than 1.7 liter per minute, preferably at 2 liters per minute.
- If the well water is stripped at a flow rate below 1.7 liter per minute for one hour, the SF6 and SF5CF3 partial pressures do not reach a steady state concentration in the headspace gas. The calculated partial pressures will therefore be too low (old bias in apparent age).
- If the ground water has a high excess air concentration, the headspace gas increases too rapidly and usually some gas escapes from below the stripping cell. In such a cases, a steady concentration is never reached and the calculated partial pressures will therefore be too low (old bias in apparent age).
- There is a CFC-12 blank with the new procedure. The exact area of the blank is very difficult to estimate because it varies from day to day. Therefore calculated concentrations CFC-12 below 50 pptv are unreliable.
- As a result, the tracer concentrations are integrated over the one hour sampling. If the tracer compositions in the ground water changed during the sampling, then the concentration determined for the tracer by the new method represents the average concentration during the hour of sampling. With the standard SF6 and CFC-12 procedures, the concentration measured is the concentration of the tracer at the instant of sampling of the ground water.
- The lower dating limit by the new method is about 40 years. All model ages of 40 years may be significantly greater than 40 years and are indicated in the Excel worksheet as 40 years or older.
- The concentrations of some of the new tracers used in the new procedure are extremely low. For example the concentration of SF5CF3 is 120 parts per quadrillion in 2007 air and was only a few parts per quadrillion in 40 year-old air. There are only about 300 molecules of SF5CF3 per cm3 of water at equilibrium with air in 40 year old ground water. This same cm3 of water contains 3.41022 molecules of water. The analytical procedure is difficuliable results are obtained by the new procedure when the volume of the stripped gas sample is greater than 80 milliliters. We often obtain two stripped gas samples per well. In some cases, the duplicate sample was less than 90 milliliters. Regardless of the volume of the stripped gas, both samples will be analyzed. The results for samples of less than 80 milliliters of stripped gas are shown in red in the "AGES" sheet of the Excel worksheet. The data in red should be considered as not reliable.
- Rarely an interfering peak elutes just before the SF5CF3 peak. When this interfering peak is large, it becomes difficult to impossible to quantify the SF5CF3 peak. The interfering peak occurs often in ground water samples that are reducing and also contain excess nitrogen produced by the denitrification of nitrates.
- The CFC-13 calculated piston flow (PF) ages appear to be useful in the vast majority of samples. Apparently, CFC-13 can be present as a trace impurity in some CFC-12 and possibly some other CFC formulations. In a few cases, we have found higher than expected concentrations of CFC-13 in samples that were highly contaminated with CFC-12 and rarely in some samples that were highly contaminated with other CFCs. In samples that are "highly" contaminated with CFC-12, the CFC-13 often gives apparent model ages that are too young.
- CFC-13 appears to degrade slightly under very reducing (methanogenic) conditions. The observed extent of degradation is CFC-11 > CFC-113 > CFC-12 > CFC-13.
- Wells that discharge water at less than 1.7 liter/min through the stripping cell can be used if the stripping time is increased. The best results are obtained when at least 100 liters of water is stripped.
Other Considerations
Shelf Life of SF5CF3 Samples
No changes in concentrations of SF5CF3 were observed after storage of 2 months.
Modification of SF5CF3 by Microbial Activity
In the microcosm experiments under anaerobic conditions, the concentrations of SF6 and SF5CF3 do not differ from the controls. The results indicate that SF6 and SF5CF3 did not degrade under anaerobic conditions.
Unsaturated Zone Processes
When the unsaturated zone is relatively thin, the unsaturated-zone air composition tracks that of the troposphere. It is reasonable to assume unsaturated-zone tracer concentrations closely track tropospheric concentrations to unsaturated zone depths of less than 10 m, in deeper unsaturated zones, there is a lag time for diffusive transport of tracers through the unsaturated zone. The time lag is largely a function of the tracer diffusion coefficients, tracer solubility in water, and soil water content (Weeks et al., 1982; Cook and Solomon, 1995).
Dissolved Gases in Ground Water
It is recommended that dissolved gas analyses be performed to determine the recharge temperature and the amount of excess air present in the ground waters.
Recharge Temperature
Uncertainty in recharge temperature of 1 to 2°C introduces an error in the SF5CF3 and SF6 model ground water ages of ±1 to 2 year because of the rapid increase in the atmospheric mixing ratios. However, a similar temperature uncertainty can introduce a significant CFC-12 ground-water age error for waters that recharge after 1990.
Excess Air
Excess air is introduced into ground water when air bubbles dissolve during a rapid rise of the water table. The addition of excess air to ground water increases the concentrations of the tracers in the groundwater above the air-water equilibrium concentration. If the presence of excess air is not considered in the calculation of model ages, then the apparent age will be too young. If the excess air present in the ground water was not known, and was underestimated by one cm3/kg of water at STP for typical U.S. ground waters, the age of the ground water will be under estimated by 1 to 2.5 years. In all cases, the error in the apparent date of recharge was higher for the waters that were recharged at the higher temperature. Excess air concentrations of 0 to about 2 cm3 per liter were found from N2-Ar measurements on shallow groundwater recharged by aerial infiltration through sandy soils, however, concentrations as high as 10 cm3 per liter can be found in ground waters from some semi-arid regions.